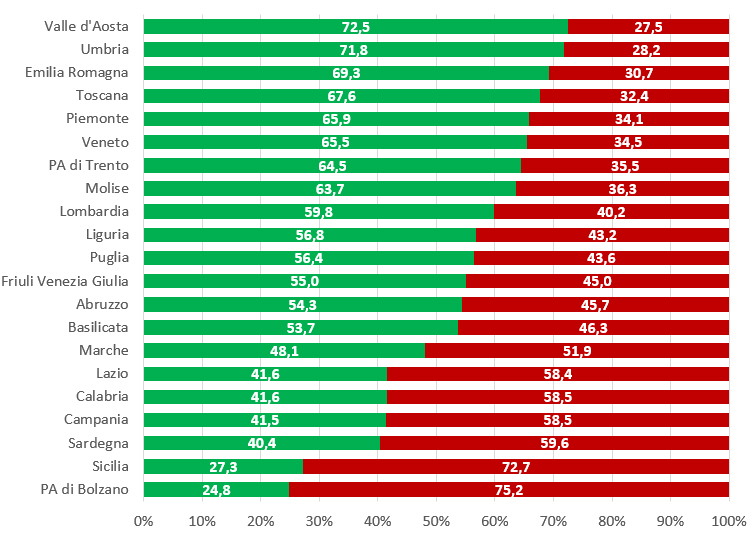
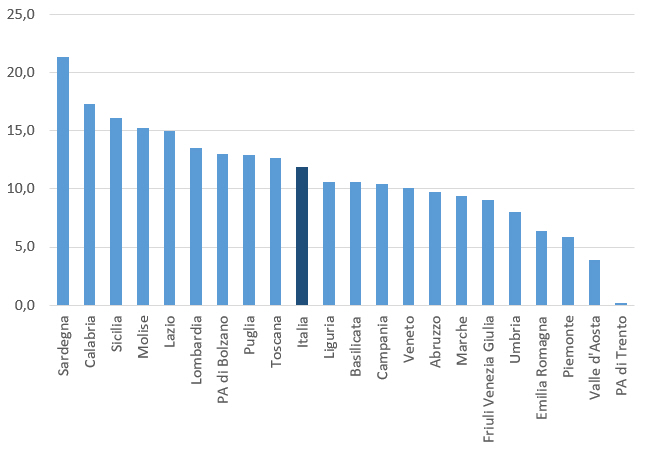
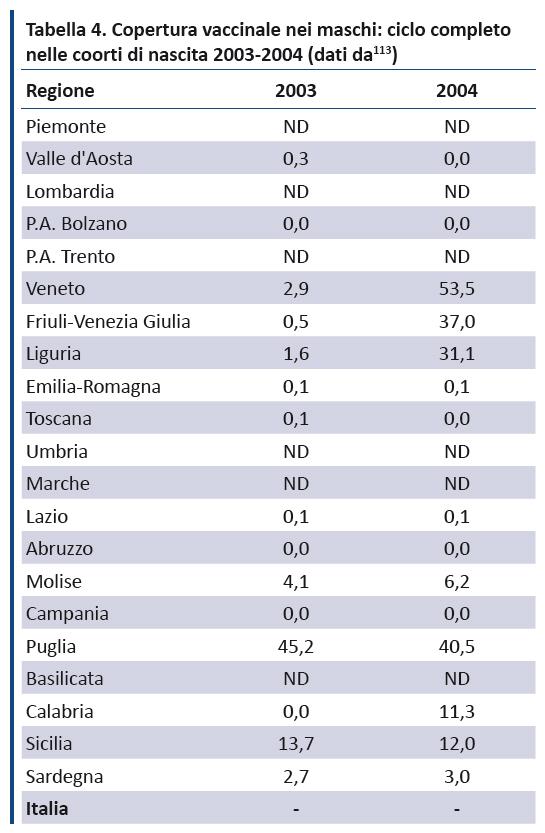
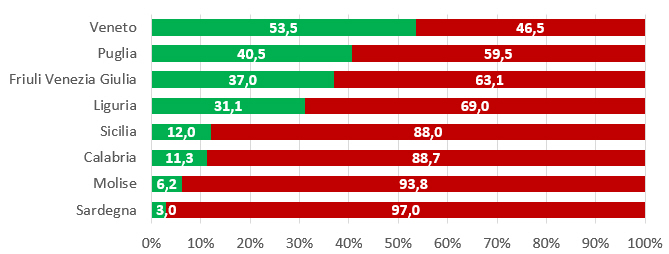
Vaccino anti-HPV: prove di efficacia, profilo di sicurezza e copertura vaccinale in Italia
Position Statement GIMBE
Vaccino anti-HPV: prove di efficacia, profilo di sicurezza e copertura vaccinale in Italia
Fondazione GIMBEEvidence 2018;10(7): e1000184 doi: 10.4470/E1000184
Pubblicato: 9 luglio 2018
Copyright: © 2018 Fondazione GIMBE Questo è un articolo open-access, distribuito con licenza Creative Commons Attribution, che ne consente lâutilizzo, la distribuzione e la riproduzione su qualsiasi supporto esclusivamente per fini non commerciali, a condizione di riportare sempre autore e citazione originale.
1. Ministero della Salute. Vaccinazione contro il Papillomavirus umano. Disponibile a: www.salute.gov.it/portale/salute/p1_5.jsp?id=31&area=Vaccinazioni. Ultimo accesso 9 luglio 2018
2. Associazione Italiana di Oncologia Medica (AIOM). Linee guida. Neoplasie dellâutero: endometrio e cervice. Novembre 2017.
3. Kuhdari P, Previato S, Giordani M, Biavati P, Ferretti S, Gabutti G. The burden of HPV-related diseases in Italy, 2001-12. J Public Health (Oxf) 2017;39:730-737.
4. Sanders GD, Taira AV. Cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003;9:37.
5. Goldie SJ, Kohli M, Grima D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004;96:604.
6. Kulasingam SL, Myers ER. Potential health and economic impact of adding a human papillomavirus vaccine to screening programs. JAMA 2003;290:781.
7. Chesson HW, et al. Cost effectiveness models of HPV vaccines. May 9, 2006 - 2006 National STD Prevention Conference.
8. Westra TA, Rozenbaum MH, Rogoza RM, et al. Until which age should women be vaccinated against HPV infection? Recommendation based on cost-effectiveness analyses. J Infect Dis 2011;204:377.
9. Drolet M, Bénard Ã, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015;15:565.
10. Favato G, Baio G, Capone A, et al.Novel health economic evaluation of a vaccination strategy to prevent HPV-related diseases: the BEST study. Med Care 2012;50:1076-85.
11. Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008;359:821.
12. Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis 2010;10:845.
13. Chesson HW, Ekwueme DU, Saraiya M, et al. The cost-effectiveness of male HPV vaccination in the United States. Vaccine 2011;29:8443.
14. Hughes JP, Garnett GP, Koutsky L. The theoretical population-level impact of a prophylactic human papilloma virus vaccine. Epidemiology 2002;13:631.
15. Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis 2004;10:1915.
16. Bogaards JA, Wallinga J, Brakenhoff RH, et al. Direct benefit of vaccinating boys along with girls against oncogenic human papillomavirus: bayesian evidence synthesis. BMJ 2015;350:h2016.
17. Elfström KM, Lazzarato F, Franceschi S, et al. Human Papillomavirus Vaccination of Boys and Extended Catch-up Vaccination: Effects on the Resilience of Programs. J Infect Dis 2016;213:199.
18. Newall AT, Beutels P, Wood JG, et al. Cost-effectiveness analyses of human papillomavirus vaccination. Lancet Infect Dis 2007;7:289.
19. Baio G, Capone A, Marcellusi A, et al. Economic burden of human papillomavirus-related diseases in Italy. PLoSOne 2012;7(11):e49699.
20. Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015;386:2078.
21. Piano Nazionale Prevenzione Vaccinale - PNPV 2017-2019. Disponibile a: www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf. Ultimo accesso: 9 luglio 2018
22. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older - United States, 2018. MMWR Morb Mortal Wkly Rep 2018; 67:158.
23. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014;63:1.
24. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015;64:300.
25. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger - United States, 2018. MMWR Morb Mortal Wkly Rep 2018;67:156.
26. Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis 2016; 16:1154.
27. Skinner SR, Szarewski A, Romanowski B, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 4-year interim follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet 2014;384:2213.
28. Castellsagué X, Muñoz N, Pitisuttithum P, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24-45 years of age. Br J Cancer 2011;105:28.
29. Luna J, Plata M, Gonzalez M, et al. Long-term follow-up observation of the safety, immunogenicity, and effectiveness of Gardasil⢠in adult women. PLoS One 2013 8:e83431.
30. Immunization Expert Work Group, Committee on Adolescent Health Care. Committee Opinion No. 704: Human Papillomavirus Vaccination. ObstetGynecol 2017; 129:e173.
31. American Academy of Family Physicians. Human papillomavirus vaccine (HPV). Disponibile a: www.aafp.org/patient-care/public-health/immunizations/disease-population/hpv.html#Recommendations. Ultimo accesso: 9 luglio 2018
32. American Academy of Pediatrics. Human Papillomaviruses. In: Red Book: 2015 Report of the Committee on Infectious Diseases, 30th edition, Kimberlin DW, Brady MT, Jackson MA, Long SS (Eds), American Academy of Pediatrics, Elk Grove Village, IL 2015. p.576.
33. Saslow D, Andrews KS, Manassaram-Baptiste D, et al. Human papillomavirus vaccination guideline update: American Cancer Society guideline endorsement. CA Cancer J Clin 2016; 66:375.
34. International Papillomavirus Society (IPVS). Disponibile a: http://ipvsoc.org. Ultimo accesso: 9 luglio 2018
35. Arrossi S, Temin S, Garland S, et al. Primary prevention of cervical cancer: American Society of Clinical Oncology resource-stratified guideline. March 17, 2017. Disponibile a: www.asco.org/practice-guidelines/quality-guidelines/guidelines/resource-stratified#/24681. Ultimo accesso: 9 luglio 2018
36. Human papillomavirus vaccines: WHO position paper, May 2017. Disponibile a: http://apps.who.int/iris/bitstream/10665/255353/1/WER9219.pdf?ua=1.Ultimo accesso: 9 luglio 2018
37. Ministero della Salute. Direzione Generale della Prevenzione Sanitaria. Circolare 24 aprile 2014 Aggiornamento della schedula vaccinale anti-papillomavirus e delle modalità di rilevazione delle coperture vaccinali. Disponibile a: www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=0&codLeg=49024&parte=1%20&serie=. Ultimo accesso: 9 luglio 2018
38. Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13-17 years--United States, 2011. MMWR Morb Mortal Wkly Rep 2012;61:671.
39. Ministero della Salute. Direzione Generale della Prevenzione Sanitaria. Circolare 9 marzo 2017. Aspetti operativi per la piena e uniforme implementazione del nuovo PNPV 2017-2019 e del relativo Calendario Vaccinale. Disponibile a: www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2017&codLeg=58583&parte=1%20&serie=null. Ultimo accesso: 9 luglio 2018
40. Reusser NM, Downing C, Guidry J, Tyring SK. HPV Carcinomas in Immunocompromised Patients. J Clin Med. 2015 Jan 29;4(2):260-81.
41. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014;58:309.
42. Strategic Advisory Group of Experts on Immunization (SAGE) of the World Health Organization. Summary of the SAGE April 2014 meeting. Disponibile a: www.who.int/immunization/sage/meetings/2014/april/report_summary_april_2014/en. Ultimo accesso: 9 luglio 2018
43. Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013;309:1793.
44. Sankaranarayanan R, Prabhu PR, Pawlita M, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol 2016;17:67.
45. Puthanakit T, Huang LM, Chiu CH, et al. Randomized Open Trial Comparing 2-Dose Regimens of the Human Papillomavirus 16/18 AS04-Adjuvanted Vaccine in Girls Aged 9-14 Years Versus a 3-Dose Regimen in Women Aged 15-25 Years. J Infect Dis 2016;214:525.
46. Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-Valent HPV Vaccine Using 2-Dose Regimens in Girls and Boys vs a 3-Dose Regimen in Women. JAMA 2016;316:2411.
47. Ogilvie G, Sauvageau C, Dionne M, et al. Immunogenicity of 2 vs 3 Doses of the Quadrivalent Human Papillomavirus Vaccine in Girls Aged 9 to 13 Years After 60 Months. JAMA 2017;317:1687.
48. Wilkin T, Lee JY, Lensing SY, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis 2010;202:1246.
49. Kahn JA, Xu J, Kapogiannis BG, et al. Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV-infected young women. Clin Infect Dis 2013; 57:735.
50. Kojic EM, Kang M, Cespedes MS, et al. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin Infect Dis 2014;59:127.
51. Levin MJ, Moscicki AB, Song LY, et al. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune DeficSyndr 2010;55:197.
52. Kosalaraksa P, Mehlsen J, Vesikari T, et al. An open-label, randomized study of a 9-valent human papillomavirus vaccine given concomitantly with diphtheria, tetanus, pertussis and poliomyelitis vaccines to healthy adolescents 11-15 years of age. Pediatr Infect Dis J 2015;34:627.
53. Schilling A, Parra MM, Gutierrez M, et al. Coadministration of a 9-Valent Human Papillomavirus Vaccine With Meningococcal and Tdap Vaccines. Pediatrics 2015;136:e563.
54. GlaxoSmithKline Vaccine HPV-007 Study Group, Romanowski B, de Borba PC, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009; 374:1975.
55. Vesikari T, Brodszki N, van Damme P, et al. A Randomized, Double-Blind, Phase III Study of the Immunogenicity and Safety of a 9-Valent Human Papillomavirus L1 Virus-Like Particle Vaccine (V503) Versus Gardasil® in 9-15-Year-Old Girls. Pediatr Infect Dis J 2015; 34:992.
56. Gardasil 9 (Human papillomavirus 9-valent vaccine, recombinant. US FDA approved product information; Whitehouse Station, NJ: Merck & Co, Inc. December 2014.
57. Petäjä T, Keränen H, Karppa T, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10-18 years. J Adolesc Health 2009; 44:33.
58. Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26:201.
59. Lin SW, Ghosh A, Porras C, et al. HPV16 seropositivity and subsequent HPV16 infection risk in a naturally infected population: comparison of serological assays. PLoS One 2013;8:e53067.
60. Safaeian M, Porras C, Schiffman M, et al. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst 2010;102:1653.
61. Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928.
62. Van Damme P, Olsson SE, Block S, et al. Immunogenicity and Safety of a 9-Valent HPV Vaccine. Pediatrics 2015; 136:e28.
63. Human Papillomavirus Bivalent (Types 16 and 18) Vaccine, Recombinant Vaccines and Related Biological Products Advisory Committee (VRBPAC) Briefing Document, September 9, 2009. Disponibile a: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM181371.pdf. Ultimo accesso: 9 luglio 2018
64. Sow PS, Watson-Jones D, Kiviat N, et al. Safety and immunogenicity of human papillomavirus-16/18 AS04-adjuvanted vaccine: a randomized trial in 10-25-year-old HIV-Seronegative African girls and young women. J Infect Dis 2013;207:1753.
65. Pedersen C, Petaja T, Strauss G, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health 2007;40:564.
66. Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin 2009;5:705.
67. Paavonen J, Naud P, Salmerón J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009;374:301.
68. Hildesheim A, Wacholder S, Catteau G, et al. Efficacy of the HPV-16/18 vaccine: final according to protocol results from the blinded phase of the randomized Costa Rica HPV-16/18 vaccine trial. Vaccine 2014;32:5087.
69. FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007;356:1915.
70. Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015;372:711.
71. Brotherton JM, Fridman M, May CL, et al. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011; 377:2085.
72. Crowe E, Pandeya N, Brotherton JM, et al. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. BMJ 2014; 348:g1458.
73. Baldur-Felskov B, Dehlendorff C, Munk C, Kjaer SK. Early impact of human papillomavirus vaccination on cervical neoplasia--nationwide follow-up of young Danish women. J Natl Cancer Inst 2014; 106:djt460.
74. Smith LM, Strumpf EC, Kaufman JS, et al. The early benefits of human papillomavirus vaccination on cervical dysplasia and anogenital warts. Pediatrics 2015; 135:e1131.
75. Hofstetter AM, Ompad DC, Stockwell MS, et al. Human Papillomavirus Vaccination and Cervical Cytology Outcomes Among Urban Low-Income Minority Females. JAMA Pediatr 2016; 170:445.
76. Garland SM, Kjaer SK, Muñoz N, et al. Impact and Effectiveness of the Quadrivalent Human Papillomavirus Vaccine: A Systematic Review of 10 Years of Real-world Experience. Clin Infect Dis 2016; 63:519.
77. Benard VB, Castle PE, Jenison SA, et al. Population-Based Incidence Rates of Cervical Intraepithelial Neoplasia in the Human Papillomavirus Vaccine Era. JAMA Oncol 2017;3:833-837.
78. Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018 May 9.
79. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011;364:401.
80. Kreimer AR, González P, Katki HA, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol 2011; 12:862.
81. Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One 2013; 8:e68329.
82. Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J ClinOncol 2018; 36:262.
83. Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis 2012; 206:1645.
84. Donovan B, Franklin N, Guy R, et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis 2011; 11:39.
85. Read TR, Hocking JS, Chen MY, et al. The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sex Transm Infect 2011; 87:544.
86. Blomberg M, Dehlendorff C, Munk C, Kjaer SK. Strongly decreased risk of genital warts after vaccination against human papillomavirus: nationwide follow-up of vaccinated and unvaccinated girls in Denmark. Clin Infect Dis 2013; 57:929.
87. Bauer HM, Wright G, Chow J. Evidence of human papillomavirus vaccine effectiveness in reducing genital warts: an analysis of California public family planning administrative claims data, 2007-2010. Am J Public Health 2012; 102:833.
88. Flagg EW, Schwartz R, Weinstock H. Prevalence of anogenital warts among participants in private health plans in the United States, 2003-2010: potential impact of human papillomavirus vaccination. Am J Public Health 2013; 103:1428.
89. Novakovic D, Cheng ATL, Zurynski Y, et al. A Prospective Study of the Incidence of Juvenile-Onset Recurrent Respiratory Papillomatosis After Implementation of a National HPV Vaccination Program. J Infect Dis 2018;217:208.
90. Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:89.
91. Huh WK, Joura EA, Giuliano AR, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet 2017;390:2143.
92. Kjaer SK, Nygård M, Dillner J, et al. A 12-Year Follow-up on the Long-Term Effectiveness of the Quadrivalent Human Papillomavirus Vaccine in 4 Nordic Countries. Clin Infect Dis 2018;66:339.
93. Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine 2009;27:5612.
94. Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum VaccinImmunother 2014;10:2147.
95. Ferris D, Samakoses R, Block SL, et al. Long-term study of a quadrivalent human papillomavirus vaccine. Pediatrics 2014;134:e657.
96. Toh ZQ, Russell FM, Reyburn R, et al. Sustained Antibody Responses 6 Years Following 1, 2, or 3 Doses of Quadrivalent Human Papillomavirus (HPV) Vaccine in Adolescent Fijian Girls, and Subsequent Responses to a Single Dose of Bivalent HPV Vaccine: A Prospective Cohort Study. Clin Infect Dis 2017;64:852.
97. Frazer IH, Cox JT, Mayeaux EJ Jr, et al. Advances in prevention of cervical cancer and other human papillomavirus-related diseases. Pediatr Infect Dis J 2006;25:S65.
98. World Health Organization. Global Advisory Committee on Vaccine Safety statement on the continued safety of HPV vaccination. March 12, 2014. Disponibile a: www.who.int/vaccine_safety/committee/topics/hpv/GACVS_Statement_HPV_12_Mar_2014.pdf. Ultimo accesso: 9 luglio 2018
99. World Health Organization. Immunization safety surveillance: guidelines for immunization programme managers on surveillance of adverse events following immunization. 3rdedition, 2015. Disponibile a: http://iris.wpro.who.int/handle/10665.1/12620.Ultimo accesso: 9 luglio 2018
100. Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009;302:750.
101. Centers for Disease Control and Prevention (CDC). Syncope after vaccination--United States, January 2005-July 2007. MMWR Morb Mortal Wkly Rep 2008;57:457.
102. Klein NP, Hansen J, Chao C, et al. Safety of quadrivalent human papillomavirus vaccine administered routinely to females. Arch PediatrAdolesc Med 2012;166:1140.
103. Human Papillomavirus Vaccination Coverage Among Adolescent Girls, 2007â2012, and Postlicensure Vaccine Safety Monitoring, 2006â2013 â United States. MMWR Recomm Rep 2013; 62:591.
104. Gee J, Naleway A, Shui I, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine 2011;29:8279.
105. Scheller NM, Pasternak B, Svanström H, Hviid A. Quadrivalent human papillomavirus vaccine and the risk of venous thromboembolism. JAMA 2014;312:187.
106. Brotherton JM, Gold MS, Kemp AS, et al. Anaphylaxis following quadrivalent human papillomavirus vaccination. CMAJ 2008;179:525.
107. Douglas RJ. Anaphylaxis following quadrivalent human papillomavirus vaccination - even safer than it appears. CMAJ 2008;179:525.
108. Kang LW, Crawford N, Tang ML, et al. Hypersensitivity reactions to human papillomavirus vaccine in Australian schoolgirls: retrospective cohort study. BMJ 2008;337:a2642.
109. Scheller NM, Svanström H, Pasternak B, et al. Quadrivalent HPV vaccination and risk of multiple sclerosis and other demyelinating diseases of the central nervous system. JAMA 2015;313:54.
110. Moreira ED Jr, Block SL, Ferris D, et al. Safety Profile of the 9-Valent HPV Vaccine: A Combined Analysis of 7 Phase III Clinical Trials. Pediatrics. 2016;138. pii: e20154387.
111. Agenzia Italiana del Farmaco. Rapporto sulla sorveglianza postmarketing dei vaccini in Italia. Disponibile a: www.agenziafarmaco.gov.it/content/rapporto-sulla-sorveglianza-postmarketing-dei-vaccini-italia. Ultimo accesso: 9 luglio 2018
112. Agenzia Italiana del Farmaco. Rapporto Vaccini 2017. La sorveglianza postmarketing in Italia. Luglio 2018: pag 57-62. Disponibile a: www.agenziafarmaco.gov.it/content/rapporto-vaccini-2017-sorveglianza-postmarketing-italia. Ultimo accesso: 9 luglio 2018
113. Ministero della Salute. Vaccinazione contro il papilloma virus (HPV) - Coperture vaccinali. Disponibile a: www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=27. Ultimo accesso: 9 luglio 2018
114. Ministero della Salute. Direzione Generale della Prevenzione Sanitaria. Coperture vaccinali al 31/12/2016 per HPV. Aggiornamento 28 dicembre 2017. Disponibile a: www.salute.gov.it/imgs/C_17_tavole_27_allegati_iitemAllegati_0_fileAllegati_itemFile_1_file.pdf. Ultimo accesso: 9 luglio 2018
115. National HPV vaccination program register. Coverage Data. Australia. Disponibile a: www.hpvregister.org.au/research/coverage-data. Ultimo accesso: 9 luglio 2018
116. Vaccine uptake guidance and the latest coverage data. Public Health England. Disponibilea: www.gov.uk/government/collections/vaccine-uptake. Ultimo accesso: 9 luglio 2018
117. Widgren K, Simonsen J, Valentiner-Branth P, Mølbak K. Uptake of the human papillomavirus-vaccination within the free-of-charge childhood vaccination programme in Denmark. Vaccine 2011;29:9663.
118. Jeyarajah J, Elam-Evans LD, Stokley S, et al. Human Papillomavirus Vaccination Coverage Among Girls Before 13 Years: A Birth Year Cohort Analysis of the National Immunization Survey-Teen, 2008-2013. ClinPediatr (Phila) 2016;55:904.
119. Walker TY, Elam-Evans LD, Singleton JA, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years - United States, 2016. MMWR Morb Mortal Wkly Rep 2017;66:874.
120. Napolitano F, Navaro M, Vezzosi L, Santagati G, Angelillo IF. Primary care pediatriciansâ attitudes and practice towards HPV vaccination: A nationwide survey in Italy. PLoS One 2018;13:e0194920.
121. Liuccio M, Borgia C, Cannistrà L, Martino B. HPV information campaigns in Italy since 2004. Ann Ig 2016;28:218-26.
122. Niccolai LM, Hansen CE. Practice- and Community-Based Interventions to Increase Human Papillomavirus Vaccine Coverage: A Systematic Review. JAMA Pediatr 2015;169:686.
123. Schuler CL, Reiter PL, Smith JS, Brewer NT. Human papillomavirus vaccine and behavioural disinhibition. Sex Transm Infect 2011;87:349.
124. Marlow LA, Forster AS, Wardle J, Waller J. Mothersâ and adolescentsâ beliefs about risk compensation following HPV vaccination. J Adolesc Health 2009;44:446.
125. Bednarczyk RA, Davis R, Ault K, et al. Sexual activity-related outcomes after human papillomavirus vaccination of 11- to 12-year-olds. Pediatrics 2012;130:798.
126. Smith LM, Kaufman JS, Strumpf EC, Lévesque LE. Effect of human papillomavirus (HPV) vaccination on clinical indicators of sexual behaviour among adolescent girls: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ 2015; 187:E74.
127. Jena AB, Goldman DP, Seabury SA. Incidence of sexually transmitted infections after human papillomavirus vaccination among adolescent females. JAMA Intern Med 2015;175:617.
128. Glasziou P, Haynes B. The paths from research to improved health outcomes. ACP J Club. 2005;142(2):A8-10.
2. Associazione Italiana di Oncologia Medica (AIOM). Linee guida. Neoplasie dellâutero: endometrio e cervice. Novembre 2017.
3. Kuhdari P, Previato S, Giordani M, Biavati P, Ferretti S, Gabutti G. The burden of HPV-related diseases in Italy, 2001-12. J Public Health (Oxf) 2017;39:730-737.
4. Sanders GD, Taira AV. Cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003;9:37.
5. Goldie SJ, Kohli M, Grima D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004;96:604.
6. Kulasingam SL, Myers ER. Potential health and economic impact of adding a human papillomavirus vaccine to screening programs. JAMA 2003;290:781.
7. Chesson HW, et al. Cost effectiveness models of HPV vaccines. May 9, 2006 - 2006 National STD Prevention Conference.
8. Westra TA, Rozenbaum MH, Rogoza RM, et al. Until which age should women be vaccinated against HPV infection? Recommendation based on cost-effectiveness analyses. J Infect Dis 2011;204:377.
9. Drolet M, Bénard Ã, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015;15:565.
10. Favato G, Baio G, Capone A, et al.Novel health economic evaluation of a vaccination strategy to prevent HPV-related diseases: the BEST study. Med Care 2012;50:1076-85.
11. Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008;359:821.
12. Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis 2010;10:845.
13. Chesson HW, Ekwueme DU, Saraiya M, et al. The cost-effectiveness of male HPV vaccination in the United States. Vaccine 2011;29:8443.
14. Hughes JP, Garnett GP, Koutsky L. The theoretical population-level impact of a prophylactic human papilloma virus vaccine. Epidemiology 2002;13:631.
15. Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis 2004;10:1915.
16. Bogaards JA, Wallinga J, Brakenhoff RH, et al. Direct benefit of vaccinating boys along with girls against oncogenic human papillomavirus: bayesian evidence synthesis. BMJ 2015;350:h2016.
17. Elfström KM, Lazzarato F, Franceschi S, et al. Human Papillomavirus Vaccination of Boys and Extended Catch-up Vaccination: Effects on the Resilience of Programs. J Infect Dis 2016;213:199.
18. Newall AT, Beutels P, Wood JG, et al. Cost-effectiveness analyses of human papillomavirus vaccination. Lancet Infect Dis 2007;7:289.
19. Baio G, Capone A, Marcellusi A, et al. Economic burden of human papillomavirus-related diseases in Italy. PLoSOne 2012;7(11):e49699.
20. Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015;386:2078.
21. Piano Nazionale Prevenzione Vaccinale - PNPV 2017-2019. Disponibile a: www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf. Ultimo accesso: 9 luglio 2018
22. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older - United States, 2018. MMWR Morb Mortal Wkly Rep 2018; 67:158.
23. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014;63:1.
24. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015;64:300.
25. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger - United States, 2018. MMWR Morb Mortal Wkly Rep 2018;67:156.
26. Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis 2016; 16:1154.
27. Skinner SR, Szarewski A, Romanowski B, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 4-year interim follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet 2014;384:2213.
28. Castellsagué X, Muñoz N, Pitisuttithum P, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24-45 years of age. Br J Cancer 2011;105:28.
29. Luna J, Plata M, Gonzalez M, et al. Long-term follow-up observation of the safety, immunogenicity, and effectiveness of Gardasil⢠in adult women. PLoS One 2013 8:e83431.
30. Immunization Expert Work Group, Committee on Adolescent Health Care. Committee Opinion No. 704: Human Papillomavirus Vaccination. ObstetGynecol 2017; 129:e173.
31. American Academy of Family Physicians. Human papillomavirus vaccine (HPV). Disponibile a: www.aafp.org/patient-care/public-health/immunizations/disease-population/hpv.html#Recommendations. Ultimo accesso: 9 luglio 2018
32. American Academy of Pediatrics. Human Papillomaviruses. In: Red Book: 2015 Report of the Committee on Infectious Diseases, 30th edition, Kimberlin DW, Brady MT, Jackson MA, Long SS (Eds), American Academy of Pediatrics, Elk Grove Village, IL 2015. p.576.
33. Saslow D, Andrews KS, Manassaram-Baptiste D, et al. Human papillomavirus vaccination guideline update: American Cancer Society guideline endorsement. CA Cancer J Clin 2016; 66:375.
34. International Papillomavirus Society (IPVS). Disponibile a: http://ipvsoc.org. Ultimo accesso: 9 luglio 2018
35. Arrossi S, Temin S, Garland S, et al. Primary prevention of cervical cancer: American Society of Clinical Oncology resource-stratified guideline. March 17, 2017. Disponibile a: www.asco.org/practice-guidelines/quality-guidelines/guidelines/resource-stratified#/24681. Ultimo accesso: 9 luglio 2018
36. Human papillomavirus vaccines: WHO position paper, May 2017. Disponibile a: http://apps.who.int/iris/bitstream/10665/255353/1/WER9219.pdf?ua=1.Ultimo accesso: 9 luglio 2018
37. Ministero della Salute. Direzione Generale della Prevenzione Sanitaria. Circolare 24 aprile 2014 Aggiornamento della schedula vaccinale anti-papillomavirus e delle modalità di rilevazione delle coperture vaccinali. Disponibile a: www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=0&codLeg=49024&parte=1%20&serie=. Ultimo accesso: 9 luglio 2018
38. Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13-17 years--United States, 2011. MMWR Morb Mortal Wkly Rep 2012;61:671.
39. Ministero della Salute. Direzione Generale della Prevenzione Sanitaria. Circolare 9 marzo 2017. Aspetti operativi per la piena e uniforme implementazione del nuovo PNPV 2017-2019 e del relativo Calendario Vaccinale. Disponibile a: www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2017&codLeg=58583&parte=1%20&serie=null. Ultimo accesso: 9 luglio 2018
40. Reusser NM, Downing C, Guidry J, Tyring SK. HPV Carcinomas in Immunocompromised Patients. J Clin Med. 2015 Jan 29;4(2):260-81.
41. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014;58:309.
42. Strategic Advisory Group of Experts on Immunization (SAGE) of the World Health Organization. Summary of the SAGE April 2014 meeting. Disponibile a: www.who.int/immunization/sage/meetings/2014/april/report_summary_april_2014/en. Ultimo accesso: 9 luglio 2018
43. Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013;309:1793.
44. Sankaranarayanan R, Prabhu PR, Pawlita M, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol 2016;17:67.
45. Puthanakit T, Huang LM, Chiu CH, et al. Randomized Open Trial Comparing 2-Dose Regimens of the Human Papillomavirus 16/18 AS04-Adjuvanted Vaccine in Girls Aged 9-14 Years Versus a 3-Dose Regimen in Women Aged 15-25 Years. J Infect Dis 2016;214:525.
46. Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-Valent HPV Vaccine Using 2-Dose Regimens in Girls and Boys vs a 3-Dose Regimen in Women. JAMA 2016;316:2411.
47. Ogilvie G, Sauvageau C, Dionne M, et al. Immunogenicity of 2 vs 3 Doses of the Quadrivalent Human Papillomavirus Vaccine in Girls Aged 9 to 13 Years After 60 Months. JAMA 2017;317:1687.
48. Wilkin T, Lee JY, Lensing SY, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis 2010;202:1246.
49. Kahn JA, Xu J, Kapogiannis BG, et al. Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV-infected young women. Clin Infect Dis 2013; 57:735.
50. Kojic EM, Kang M, Cespedes MS, et al. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin Infect Dis 2014;59:127.
51. Levin MJ, Moscicki AB, Song LY, et al. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune DeficSyndr 2010;55:197.
52. Kosalaraksa P, Mehlsen J, Vesikari T, et al. An open-label, randomized study of a 9-valent human papillomavirus vaccine given concomitantly with diphtheria, tetanus, pertussis and poliomyelitis vaccines to healthy adolescents 11-15 years of age. Pediatr Infect Dis J 2015;34:627.
53. Schilling A, Parra MM, Gutierrez M, et al. Coadministration of a 9-Valent Human Papillomavirus Vaccine With Meningococcal and Tdap Vaccines. Pediatrics 2015;136:e563.
54. GlaxoSmithKline Vaccine HPV-007 Study Group, Romanowski B, de Borba PC, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009; 374:1975.
55. Vesikari T, Brodszki N, van Damme P, et al. A Randomized, Double-Blind, Phase III Study of the Immunogenicity and Safety of a 9-Valent Human Papillomavirus L1 Virus-Like Particle Vaccine (V503) Versus Gardasil® in 9-15-Year-Old Girls. Pediatr Infect Dis J 2015; 34:992.
56. Gardasil 9 (Human papillomavirus 9-valent vaccine, recombinant. US FDA approved product information; Whitehouse Station, NJ: Merck & Co, Inc. December 2014.
57. Petäjä T, Keränen H, Karppa T, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10-18 years. J Adolesc Health 2009; 44:33.
58. Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26:201.
59. Lin SW, Ghosh A, Porras C, et al. HPV16 seropositivity and subsequent HPV16 infection risk in a naturally infected population: comparison of serological assays. PLoS One 2013;8:e53067.
60. Safaeian M, Porras C, Schiffman M, et al. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst 2010;102:1653.
61. Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928.
62. Van Damme P, Olsson SE, Block S, et al. Immunogenicity and Safety of a 9-Valent HPV Vaccine. Pediatrics 2015; 136:e28.
63. Human Papillomavirus Bivalent (Types 16 and 18) Vaccine, Recombinant Vaccines and Related Biological Products Advisory Committee (VRBPAC) Briefing Document, September 9, 2009. Disponibile a: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM181371.pdf. Ultimo accesso: 9 luglio 2018
64. Sow PS, Watson-Jones D, Kiviat N, et al. Safety and immunogenicity of human papillomavirus-16/18 AS04-adjuvanted vaccine: a randomized trial in 10-25-year-old HIV-Seronegative African girls and young women. J Infect Dis 2013;207:1753.
65. Pedersen C, Petaja T, Strauss G, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health 2007;40:564.
66. Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin 2009;5:705.
67. Paavonen J, Naud P, Salmerón J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009;374:301.
68. Hildesheim A, Wacholder S, Catteau G, et al. Efficacy of the HPV-16/18 vaccine: final according to protocol results from the blinded phase of the randomized Costa Rica HPV-16/18 vaccine trial. Vaccine 2014;32:5087.
69. FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007;356:1915.
70. Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015;372:711.
71. Brotherton JM, Fridman M, May CL, et al. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011; 377:2085.
72. Crowe E, Pandeya N, Brotherton JM, et al. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. BMJ 2014; 348:g1458.
73. Baldur-Felskov B, Dehlendorff C, Munk C, Kjaer SK. Early impact of human papillomavirus vaccination on cervical neoplasia--nationwide follow-up of young Danish women. J Natl Cancer Inst 2014; 106:djt460.
74. Smith LM, Strumpf EC, Kaufman JS, et al. The early benefits of human papillomavirus vaccination on cervical dysplasia and anogenital warts. Pediatrics 2015; 135:e1131.
75. Hofstetter AM, Ompad DC, Stockwell MS, et al. Human Papillomavirus Vaccination and Cervical Cytology Outcomes Among Urban Low-Income Minority Females. JAMA Pediatr 2016; 170:445.
76. Garland SM, Kjaer SK, Muñoz N, et al. Impact and Effectiveness of the Quadrivalent Human Papillomavirus Vaccine: A Systematic Review of 10 Years of Real-world Experience. Clin Infect Dis 2016; 63:519.
77. Benard VB, Castle PE, Jenison SA, et al. Population-Based Incidence Rates of Cervical Intraepithelial Neoplasia in the Human Papillomavirus Vaccine Era. JAMA Oncol 2017;3:833-837.
78. Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018 May 9.
79. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011;364:401.
80. Kreimer AR, González P, Katki HA, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol 2011; 12:862.
81. Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One 2013; 8:e68329.
82. Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J ClinOncol 2018; 36:262.
83. Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis 2012; 206:1645.
84. Donovan B, Franklin N, Guy R, et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis 2011; 11:39.
85. Read TR, Hocking JS, Chen MY, et al. The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sex Transm Infect 2011; 87:544.
86. Blomberg M, Dehlendorff C, Munk C, Kjaer SK. Strongly decreased risk of genital warts after vaccination against human papillomavirus: nationwide follow-up of vaccinated and unvaccinated girls in Denmark. Clin Infect Dis 2013; 57:929.
87. Bauer HM, Wright G, Chow J. Evidence of human papillomavirus vaccine effectiveness in reducing genital warts: an analysis of California public family planning administrative claims data, 2007-2010. Am J Public Health 2012; 102:833.
88. Flagg EW, Schwartz R, Weinstock H. Prevalence of anogenital warts among participants in private health plans in the United States, 2003-2010: potential impact of human papillomavirus vaccination. Am J Public Health 2013; 103:1428.
89. Novakovic D, Cheng ATL, Zurynski Y, et al. A Prospective Study of the Incidence of Juvenile-Onset Recurrent Respiratory Papillomatosis After Implementation of a National HPV Vaccination Program. J Infect Dis 2018;217:208.
90. Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:89.
91. Huh WK, Joura EA, Giuliano AR, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet 2017;390:2143.
92. Kjaer SK, Nygård M, Dillner J, et al. A 12-Year Follow-up on the Long-Term Effectiveness of the Quadrivalent Human Papillomavirus Vaccine in 4 Nordic Countries. Clin Infect Dis 2018;66:339.
93. Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine 2009;27:5612.
94. Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum VaccinImmunother 2014;10:2147.
95. Ferris D, Samakoses R, Block SL, et al. Long-term study of a quadrivalent human papillomavirus vaccine. Pediatrics 2014;134:e657.
96. Toh ZQ, Russell FM, Reyburn R, et al. Sustained Antibody Responses 6 Years Following 1, 2, or 3 Doses of Quadrivalent Human Papillomavirus (HPV) Vaccine in Adolescent Fijian Girls, and Subsequent Responses to a Single Dose of Bivalent HPV Vaccine: A Prospective Cohort Study. Clin Infect Dis 2017;64:852.
97. Frazer IH, Cox JT, Mayeaux EJ Jr, et al. Advances in prevention of cervical cancer and other human papillomavirus-related diseases. Pediatr Infect Dis J 2006;25:S65.
98. World Health Organization. Global Advisory Committee on Vaccine Safety statement on the continued safety of HPV vaccination. March 12, 2014. Disponibile a: www.who.int/vaccine_safety/committee/topics/hpv/GACVS_Statement_HPV_12_Mar_2014.pdf. Ultimo accesso: 9 luglio 2018
99. World Health Organization. Immunization safety surveillance: guidelines for immunization programme managers on surveillance of adverse events following immunization. 3rdedition, 2015. Disponibile a: http://iris.wpro.who.int/handle/10665.1/12620.Ultimo accesso: 9 luglio 2018
100. Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009;302:750.
101. Centers for Disease Control and Prevention (CDC). Syncope after vaccination--United States, January 2005-July 2007. MMWR Morb Mortal Wkly Rep 2008;57:457.
102. Klein NP, Hansen J, Chao C, et al. Safety of quadrivalent human papillomavirus vaccine administered routinely to females. Arch PediatrAdolesc Med 2012;166:1140.
103. Human Papillomavirus Vaccination Coverage Among Adolescent Girls, 2007â2012, and Postlicensure Vaccine Safety Monitoring, 2006â2013 â United States. MMWR Recomm Rep 2013; 62:591.
104. Gee J, Naleway A, Shui I, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine 2011;29:8279.
105. Scheller NM, Pasternak B, Svanström H, Hviid A. Quadrivalent human papillomavirus vaccine and the risk of venous thromboembolism. JAMA 2014;312:187.
106. Brotherton JM, Gold MS, Kemp AS, et al. Anaphylaxis following quadrivalent human papillomavirus vaccination. CMAJ 2008;179:525.
107. Douglas RJ. Anaphylaxis following quadrivalent human papillomavirus vaccination - even safer than it appears. CMAJ 2008;179:525.
108. Kang LW, Crawford N, Tang ML, et al. Hypersensitivity reactions to human papillomavirus vaccine in Australian schoolgirls: retrospective cohort study. BMJ 2008;337:a2642.
109. Scheller NM, Svanström H, Pasternak B, et al. Quadrivalent HPV vaccination and risk of multiple sclerosis and other demyelinating diseases of the central nervous system. JAMA 2015;313:54.
110. Moreira ED Jr, Block SL, Ferris D, et al. Safety Profile of the 9-Valent HPV Vaccine: A Combined Analysis of 7 Phase III Clinical Trials. Pediatrics. 2016;138. pii: e20154387.
111. Agenzia Italiana del Farmaco. Rapporto sulla sorveglianza postmarketing dei vaccini in Italia. Disponibile a: www.agenziafarmaco.gov.it/content/rapporto-sulla-sorveglianza-postmarketing-dei-vaccini-italia. Ultimo accesso: 9 luglio 2018
112. Agenzia Italiana del Farmaco. Rapporto Vaccini 2017. La sorveglianza postmarketing in Italia. Luglio 2018: pag 57-62. Disponibile a: www.agenziafarmaco.gov.it/content/rapporto-vaccini-2017-sorveglianza-postmarketing-italia. Ultimo accesso: 9 luglio 2018
113. Ministero della Salute. Vaccinazione contro il papilloma virus (HPV) - Coperture vaccinali. Disponibile a: www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=27. Ultimo accesso: 9 luglio 2018
114. Ministero della Salute. Direzione Generale della Prevenzione Sanitaria. Coperture vaccinali al 31/12/2016 per HPV. Aggiornamento 28 dicembre 2017. Disponibile a: www.salute.gov.it/imgs/C_17_tavole_27_allegati_iitemAllegati_0_fileAllegati_itemFile_1_file.pdf. Ultimo accesso: 9 luglio 2018
115. National HPV vaccination program register. Coverage Data. Australia. Disponibile a: www.hpvregister.org.au/research/coverage-data. Ultimo accesso: 9 luglio 2018
116. Vaccine uptake guidance and the latest coverage data. Public Health England. Disponibilea: www.gov.uk/government/collections/vaccine-uptake. Ultimo accesso: 9 luglio 2018
117. Widgren K, Simonsen J, Valentiner-Branth P, Mølbak K. Uptake of the human papillomavirus-vaccination within the free-of-charge childhood vaccination programme in Denmark. Vaccine 2011;29:9663.
118. Jeyarajah J, Elam-Evans LD, Stokley S, et al. Human Papillomavirus Vaccination Coverage Among Girls Before 13 Years: A Birth Year Cohort Analysis of the National Immunization Survey-Teen, 2008-2013. ClinPediatr (Phila) 2016;55:904.
119. Walker TY, Elam-Evans LD, Singleton JA, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years - United States, 2016. MMWR Morb Mortal Wkly Rep 2017;66:874.
120. Napolitano F, Navaro M, Vezzosi L, Santagati G, Angelillo IF. Primary care pediatriciansâ attitudes and practice towards HPV vaccination: A nationwide survey in Italy. PLoS One 2018;13:e0194920.
121. Liuccio M, Borgia C, Cannistrà L, Martino B. HPV information campaigns in Italy since 2004. Ann Ig 2016;28:218-26.
122. Niccolai LM, Hansen CE. Practice- and Community-Based Interventions to Increase Human Papillomavirus Vaccine Coverage: A Systematic Review. JAMA Pediatr 2015;169:686.
123. Schuler CL, Reiter PL, Smith JS, Brewer NT. Human papillomavirus vaccine and behavioural disinhibition. Sex Transm Infect 2011;87:349.
124. Marlow LA, Forster AS, Wardle J, Waller J. Mothersâ and adolescentsâ beliefs about risk compensation following HPV vaccination. J Adolesc Health 2009;44:446.
125. Bednarczyk RA, Davis R, Ault K, et al. Sexual activity-related outcomes after human papillomavirus vaccination of 11- to 12-year-olds. Pediatrics 2012;130:798.
126. Smith LM, Kaufman JS, Strumpf EC, Lévesque LE. Effect of human papillomavirus (HPV) vaccination on clinical indicators of sexual behaviour among adolescent girls: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ 2015; 187:E74.
127. Jena AB, Goldman DP, Seabury SA. Incidence of sexually transmitted infections after human papillomavirus vaccination among adolescent females. JAMA Intern Med 2015;175:617.
128. Glasziou P, Haynes B. The paths from research to improved health outcomes. ACP J Club. 2005;142(2):A8-10.