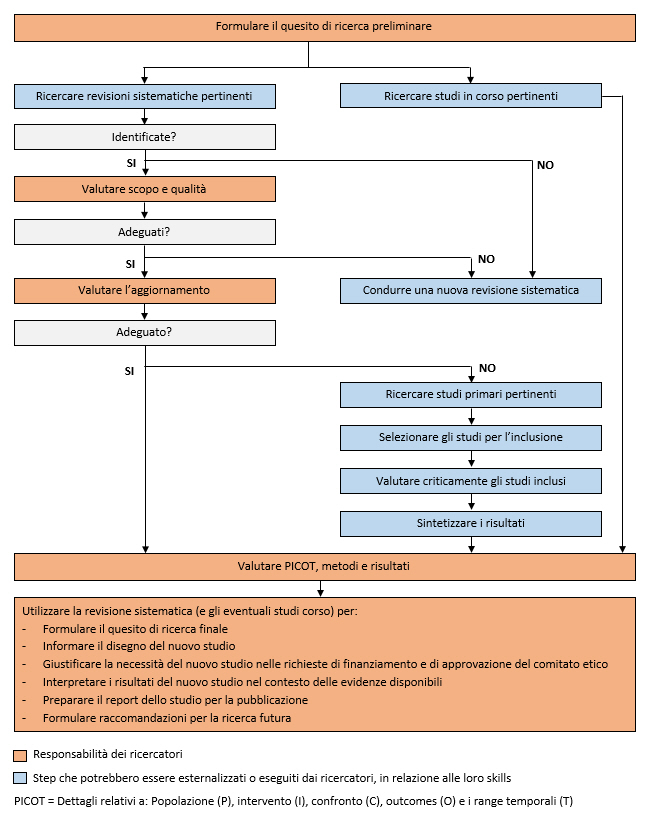
Evidence-Based Research: le revisioni sistematiche devono sempre informare i nuovi studi primari
Guidelines & Standards
Evidence-Based Research: le revisioni sistematiche devono sempre informare i nuovi studi primari
Hans Lund, Klara Brunnhuber, Carsten Juhl, Karen Robinson, Marlies Leenaars, Bertil F Dorch, Gro Jamtvedt, Monica W Nortvedt, Robin Christensen, Iain ChalmersEvidence 2017;9(3): e1000164 doi: 10.4470/E1000164
Pubblicato: 28 marzo 2017
Copyright: © 2017 Lund et al. Questo è un articolo open-access, distribuito con licenza Creative Commons Attribution, che ne consente l’utilizzo, la distribuzione e la riproduzione su qualsiasi supporto esclusivamente per fini non commerciali, a condizione di riportare sempre autore e citazione originale.
1. Young C, Horton R. Putting clinical trials into context. Lancet 2005;366:107-8.
2. Chalmers I. Academia’s failure to support systematic reviews. Lancet 2005;365:469.
3. Robinson KA, Goodman SN. A systematic examination of the citation of prior research in reports of randomized, controlled trials. Ann Intern Med 2011;154:50-5.
4. Clarke M, Chalmers I. Discussion sections in reports of controlled trials published in general medical journals: islands in search of continents? JAMA 1998;280:280-2.
5. Clarke M, Alderson P, Chalmers I. Discussion sections in reports of controlled trials published in general medical journals. JAMA 2002;287:2799-801.
6. Cooper NJ, Jones DR, Sutton AJ. The use of systematic reviews when designing studies. Clin Trials 2005;2:260-4.
7. Fergusson D, Glass KC, Hutton B, Shapiro S. Randomized controlled trials of aprotinin in cardiac surgery: could clinical equipoise have stopped the bleeding? Clin Trials 2005;2:218-29, discussion 229-32.
8. Clarke M, Hopewell S, Chalmers I. Clinical trials should begin and end with systematic reviews of relevant evidence: 12 years and waiting. Lancet 2010;376:20-1.
9. Sheth U, Simunovic N, Tornetta P 3rd, , Einhorn TA, Bhandari M. Poor citation of prior evidence in hip fracture trials. J Bone Joint Surg Am 2011;93:2079-86.
10. Habre C, Tramèr MR, Pöpping DM, Elia N. Ability of a meta-analysis to prevent redundant research: systematic review of studies on pain from propofol injection. BMJ 2014;348:g5219.
11. Sawin VI, Robinson KA. Biased and inadequate citation of prior research in reports of cardiovascular trials is a continuing source of waste in research. J Clin Epidemiol 2016;69:174-8.
12. Clarke M, Hopewell S, Chalmers I. Reports of clinical trials should begin and end with up-to-date systematic reviews of other relevant evidence: a status report. J R Soc Med 2007;100:187-90.
13. Greenberg SA. How citation distortions create unfounded authority: analysis of a citation network. BMJ 2009;339:b2680.
14. Bastiaansen JA, de Vries YA, Munafò MR. Citation distortions in the literature on the serotonin-transporter-linked polymorphic region and amygdala activation. Biol Psychiatry 2015;78:e35-6.
15. Thornley C, Watkinson A, Nicholas D, et al. The role of trust and authority in the citation behaviour of researchers. Information Research 2015;20: 677.
16. Perino AC, Hoang DD, Holmes TH, et al. Association between success rate and citation count of studies of radiofrequency catheter ablation for atrial fibrillation: possible evidence of citation bias. Circ Cardiovasc Qual Outcomes 2014;7:687-92.
17. Jannot AS, Agoritsas T, Gayet-Ageron A, Perneger TV. Citation bias favoring statistically significant studies was present in medical research. J Clin Epidemiol 2013;66:296-301.
18. Fiorentino F, Vasilakis C, Treasure T. Clinical reports of pulmonary metastasectomy for colorectal cancer: a citation network analysis. Br J Cancer 2011;104:1085-97.
19. Robinson KA. Use of prior research in the justification and interpretation of clinical trials. Johns Hopkins University, 2009.
20. National Institute for Health Research. Guidance notes for applicants: outline applications. NIHR, 2016.
21. Chalmers I. The lethal consequences of failing to make full use of all relevant evidence about the effects of medical treatments: the importance of systematic reviews. In: Rothwell PM, ed. Treating individuals—from randomised trials to personalised medicine. Lancet, 2007: 37-58.
22. Lund H, Juhl C, Christensen R. Systematic reviews and research waste. Lancet 2016;387:123-4.
23. Mahtani KR. All health researchers should begin their training by preparing at least one systematic review. J R Soc Med 2016;109:264-8.
24. Kleinert S, Benham L, Collingridge D, Summerskill W, Horton R. Further emphasis on research in context. Lancet 2014;384:2176-7.
25. Jefferson T, Deeks J. The use of systematic reviews for editorial peer reviewing: a population approach. In: Godlee F, Jefferson T, eds. Peer review in health sciences. BMJ Books, 1999: 224-34.
26. Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up?PLoS Med 2010;7:e1000326.
27. Dickersin K, Rennie D. Registering clinical trials. JAMA 2003;290:516-23.
28. Chalmers I, Glasziou P. Systematic reviews and research waste. Lancet 2016;387:122-3.
29. Macleod MR, Michie S, Roberts I, et al. Biomedical research: increasing value, reducing waste. Lancet 2014;383:101-4.
30. Ioannidis JP, Greenland S, Hlatky MA, et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014;383:166-75.
31. Glasziou P, Altman DG, Bossuyt P, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 2014;383:267-76.
32. Chan AW, Song F, Vickers A, et al. Increasing value and reducing waste: addressing inaccessible research. Lancet 2014;383:257-66.
33. Al-Shahi Salman R, Beller E, Kagan J, et al. Increasing value and reducing waste in biomedical research regulation and management. Lancet 2014;383:176-85.
34. Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet 2009;374:86-9.
35. Starr M, Chalmers I, Clarke M, Oxman AD. The origins, evolution, and future of The Cochrane Database of Systematic Reviews. Int J Technol Assess Health Care 2009;25(Suppl 1):182-95.
36. Elliott JH, Turner T, Clavisi O, et al. Living systematic reviews: an emerging opportunity to narrow the evidence-practice gap. PLoS Med 2014;11:e1001603.
2. Chalmers I. Academia’s failure to support systematic reviews. Lancet 2005;365:469.
3. Robinson KA, Goodman SN. A systematic examination of the citation of prior research in reports of randomized, controlled trials. Ann Intern Med 2011;154:50-5.
4. Clarke M, Chalmers I. Discussion sections in reports of controlled trials published in general medical journals: islands in search of continents? JAMA 1998;280:280-2.
5. Clarke M, Alderson P, Chalmers I. Discussion sections in reports of controlled trials published in general medical journals. JAMA 2002;287:2799-801.
6. Cooper NJ, Jones DR, Sutton AJ. The use of systematic reviews when designing studies. Clin Trials 2005;2:260-4.
7. Fergusson D, Glass KC, Hutton B, Shapiro S. Randomized controlled trials of aprotinin in cardiac surgery: could clinical equipoise have stopped the bleeding? Clin Trials 2005;2:218-29, discussion 229-32.
8. Clarke M, Hopewell S, Chalmers I. Clinical trials should begin and end with systematic reviews of relevant evidence: 12 years and waiting. Lancet 2010;376:20-1.
9. Sheth U, Simunovic N, Tornetta P 3rd, , Einhorn TA, Bhandari M. Poor citation of prior evidence in hip fracture trials. J Bone Joint Surg Am 2011;93:2079-86.
10. Habre C, Tramèr MR, Pöpping DM, Elia N. Ability of a meta-analysis to prevent redundant research: systematic review of studies on pain from propofol injection. BMJ 2014;348:g5219.
11. Sawin VI, Robinson KA. Biased and inadequate citation of prior research in reports of cardiovascular trials is a continuing source of waste in research. J Clin Epidemiol 2016;69:174-8.
12. Clarke M, Hopewell S, Chalmers I. Reports of clinical trials should begin and end with up-to-date systematic reviews of other relevant evidence: a status report. J R Soc Med 2007;100:187-90.
13. Greenberg SA. How citation distortions create unfounded authority: analysis of a citation network. BMJ 2009;339:b2680.
14. Bastiaansen JA, de Vries YA, Munafò MR. Citation distortions in the literature on the serotonin-transporter-linked polymorphic region and amygdala activation. Biol Psychiatry 2015;78:e35-6.
15. Thornley C, Watkinson A, Nicholas D, et al. The role of trust and authority in the citation behaviour of researchers. Information Research 2015;20: 677.
16. Perino AC, Hoang DD, Holmes TH, et al. Association between success rate and citation count of studies of radiofrequency catheter ablation for atrial fibrillation: possible evidence of citation bias. Circ Cardiovasc Qual Outcomes 2014;7:687-92.
17. Jannot AS, Agoritsas T, Gayet-Ageron A, Perneger TV. Citation bias favoring statistically significant studies was present in medical research. J Clin Epidemiol 2013;66:296-301.
18. Fiorentino F, Vasilakis C, Treasure T. Clinical reports of pulmonary metastasectomy for colorectal cancer: a citation network analysis. Br J Cancer 2011;104:1085-97.
19. Robinson KA. Use of prior research in the justification and interpretation of clinical trials. Johns Hopkins University, 2009.
20. National Institute for Health Research. Guidance notes for applicants: outline applications. NIHR, 2016.
21. Chalmers I. The lethal consequences of failing to make full use of all relevant evidence about the effects of medical treatments: the importance of systematic reviews. In: Rothwell PM, ed. Treating individuals—from randomised trials to personalised medicine. Lancet, 2007: 37-58.
22. Lund H, Juhl C, Christensen R. Systematic reviews and research waste. Lancet 2016;387:123-4.
23. Mahtani KR. All health researchers should begin their training by preparing at least one systematic review. J R Soc Med 2016;109:264-8.
24. Kleinert S, Benham L, Collingridge D, Summerskill W, Horton R. Further emphasis on research in context. Lancet 2014;384:2176-7.
25. Jefferson T, Deeks J. The use of systematic reviews for editorial peer reviewing: a population approach. In: Godlee F, Jefferson T, eds. Peer review in health sciences. BMJ Books, 1999: 224-34.
26. Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up?PLoS Med 2010;7:e1000326.
27. Dickersin K, Rennie D. Registering clinical trials. JAMA 2003;290:516-23.
28. Chalmers I, Glasziou P. Systematic reviews and research waste. Lancet 2016;387:122-3.
29. Macleod MR, Michie S, Roberts I, et al. Biomedical research: increasing value, reducing waste. Lancet 2014;383:101-4.
30. Ioannidis JP, Greenland S, Hlatky MA, et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014;383:166-75.
31. Glasziou P, Altman DG, Bossuyt P, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 2014;383:267-76.
32. Chan AW, Song F, Vickers A, et al. Increasing value and reducing waste: addressing inaccessible research. Lancet 2014;383:257-66.
33. Al-Shahi Salman R, Beller E, Kagan J, et al. Increasing value and reducing waste in biomedical research regulation and management. Lancet 2014;383:176-85.
34. Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet 2009;374:86-9.
35. Starr M, Chalmers I, Clarke M, Oxman AD. The origins, evolution, and future of The Cochrane Database of Systematic Reviews. Int J Technol Assess Health Care 2009;25(Suppl 1):182-95.
36. Elliott JH, Turner T, Clavisi O, et al. Living systematic reviews: an emerging opportunity to narrow the evidence-practice gap. PLoS Med 2014;11:e1001603.