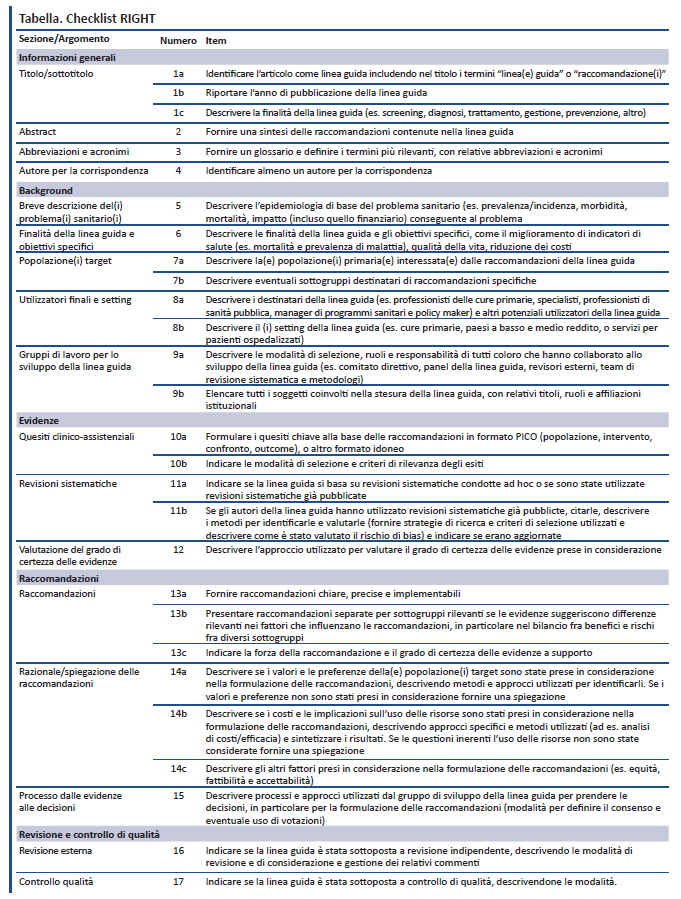
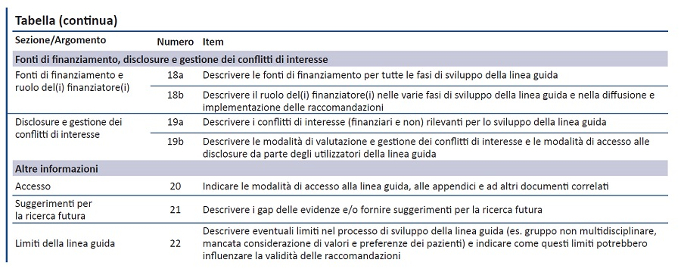
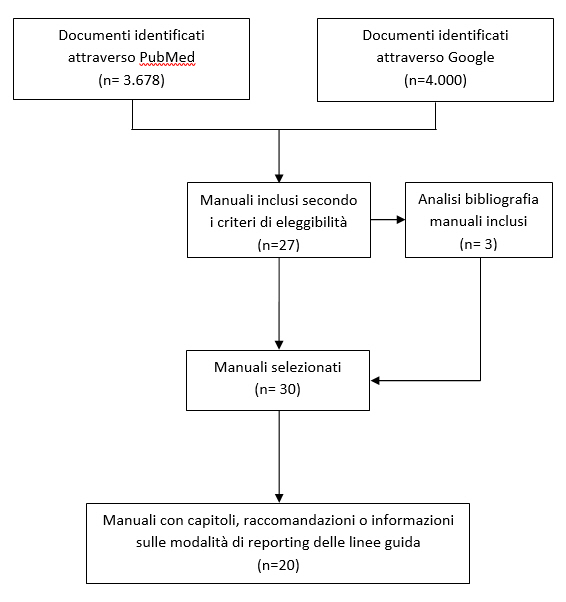
Guidelines & Standards
RIGHT statement: strumento per il reporting delle linee guida per la pratica clinica
Yaolong Chen1, Kehu Yang; Ana Marušic, Amir Qaseem, Joerg J. Meerpohl, Signe Flottorp, Elie A. Ak, Holger J. Schünemann, Edwin S.Y. Chan, Yngve Falck-Ytter, Faruque Ahmed, Sarah Barber, Chiehfeng Chen, Mingming Zhang, Bin Xu, Jinhui Tian, Fujian Song, Hongcai Shang, Kun Tang, Qi Wang, Susan L. Norris per il gruppo di lavoro RIGHT - Reporting Items for Practice Guidelines in Healthcare (i componenti del gruppo di lavoro RIGHT sono elencati nell’appendice 1 e i rispettivi contributi nell’appendice 2.)Evidence 2017;9(2): e1000161 doi: 10.4470/E1000161
Pubblicato: 23 febbraio 2017
Copyright: © 2017 Chen et al. Questo è un articolo open-access, distribuito con licenza Creative Commons Attribution, che ne consente l’utilizzo, la distribuzione e la riproduzione su qualsiasi supporto esclusivamente per fini non commerciali, a condizione di riportare sempre autore e citazione originale.
1. Oxman AD, Schünemann HJ, Fretheim A. Improving the use of research evidence in guideline development: 14. Reporting guidelines. Health Res Policy Syst 2006;4:26.
2. Grilli R, Magrini N, Penna A, Mura G, Liberati A. Practice guidelines developed by specialty societies: the need for a critical appraisal. Lancet 2000;355:103-6.
3. Shiffman RN, Shekelle P, Overhage JM, Slutsky J, Grimshaw J, Deshpande AM. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med 2003;139:493-8.
4. Oxman AD, Schünemann HJ, Fretheim A. Improving the use of research evidence in guideline development: 16. Evaluation. Health Res Policy Syst 2006;4:28.
5. Wilson KC, Irwin RS, File TM Jr, Schünemann HJ, Guyatt GH, Rabe KF; ATS/ERS Ad Hoc Committee on Integrating and Coordinating Efforts in COPD Guideline Development. Reporting and publishing guidelines: article 12 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report. Proc Am Thorac Soc 2012;9:293-7.
6. Huwiler-Müntener K, Jüni P, Junker C, Egger M. Quality of reporting of randomized trials as a measure of methodologic quality. JAMA 2002;287:2801-4.
7. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010;182:E839-42.
8. Brouwers MC, Kerkvliet K, Spithoff K; AGREE Next Steps Consortium. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ 2016;352:i1152.
9. Moher D, Schulz KF, Simera I, Altman DG. Guidance for developers of health research reporting guidelines. PLoS Med 2010;7:e1000217.
10. EQUATOR Network. Reporting items for guidelines in health systems. 2014. Disponibile a: www.equator-network.org/library/reporting-guidelines-under-development. Ultimo accesso: 23 febbraio 2017.
11. EQUATOR Network. Reporting guidelines for main study types. 2014. Disponibile a: www.equator-network.org/library. Ultimo accesso: 23 febbraio 2017.
12. Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleža-Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200-7.
13. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9.
14. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D; CARE Group. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol 2014;67:46-51.
15. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Ann Intern Med 2010;152:726-32.
16. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147: 573-7.
17. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57.
18. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al; Standards for Reporting of Diagnostic Accuracy. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Ann Intern Med 2003;138:40-4.
19. Davidoff F, Batalden P, Stevens D, Ogrinc G, Mooney SE; SQUIRE development group. Publication guidelines for quality improvement studies in health care: evolution of the SQUIRE project. BMJ 2009;338:a3152.
20. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al; CHEERS Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346:f1049.
21. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 2010;1:94-9.
22. RIGHT Statement. About RIGHT. 2014. Disponibile a: www.right-statement.org. Ultimo accesso: 23 febbraio 2017.
23. Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2:1-88.
24. Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med 2011;8:e1000393.
25. Greenland S, O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics 2001;2:463-71.
26. Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282:1054-60.
27. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al; AGREE Next Steps Consortium. The Global Rating Scale complements the AGREE II in advancing the quality of practice guidelines. J Clin Epidemiol 2012;65:526-34.
28. Grimmer K, Dizon JM, Milanese S, King E, Beaton K, Thorpe O, et al. Efficient clinical evaluation of guideline quality: development and testing of a new tool. BMC Med Res Methodol 2014;14:63.
29. Schünemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ 2014;186:E123-42.
30. Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 2014;383:267-76.
31. Ansari S, Rashidian A. Guidelines for guidelines: are they up to the task? A comparative assessment of clinical practice guideline development handbooks. PLoS One. 2012;7:e49864. [PMID:23189167] doi:10.1371/journal.pone.0049864
32. Vernooij RW, Sanabria AJ, Solà I, Alonso-Coello P, Martínez García L. Guidance for updating clinical practice guidelines: a systematic review of methodological handbooks. Implement Sci. 2014;9:3. [PMID: 24383701] doi:10.1186/1748-5908-9-3
2. Grilli R, Magrini N, Penna A, Mura G, Liberati A. Practice guidelines developed by specialty societies: the need for a critical appraisal. Lancet 2000;355:103-6.
3. Shiffman RN, Shekelle P, Overhage JM, Slutsky J, Grimshaw J, Deshpande AM. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med 2003;139:493-8.
4. Oxman AD, Schünemann HJ, Fretheim A. Improving the use of research evidence in guideline development: 16. Evaluation. Health Res Policy Syst 2006;4:28.
5. Wilson KC, Irwin RS, File TM Jr, Schünemann HJ, Guyatt GH, Rabe KF; ATS/ERS Ad Hoc Committee on Integrating and Coordinating Efforts in COPD Guideline Development. Reporting and publishing guidelines: article 12 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report. Proc Am Thorac Soc 2012;9:293-7.
6. Huwiler-Müntener K, Jüni P, Junker C, Egger M. Quality of reporting of randomized trials as a measure of methodologic quality. JAMA 2002;287:2801-4.
7. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010;182:E839-42.
8. Brouwers MC, Kerkvliet K, Spithoff K; AGREE Next Steps Consortium. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ 2016;352:i1152.
9. Moher D, Schulz KF, Simera I, Altman DG. Guidance for developers of health research reporting guidelines. PLoS Med 2010;7:e1000217.
10. EQUATOR Network. Reporting items for guidelines in health systems. 2014. Disponibile a: www.equator-network.org/library/reporting-guidelines-under-development. Ultimo accesso: 23 febbraio 2017.
11. EQUATOR Network. Reporting guidelines for main study types. 2014. Disponibile a: www.equator-network.org/library. Ultimo accesso: 23 febbraio 2017.
12. Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleža-Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200-7.
13. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9.
14. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D; CARE Group. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol 2014;67:46-51.
15. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Ann Intern Med 2010;152:726-32.
16. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147: 573-7.
17. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57.
18. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al; Standards for Reporting of Diagnostic Accuracy. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Ann Intern Med 2003;138:40-4.
19. Davidoff F, Batalden P, Stevens D, Ogrinc G, Mooney SE; SQUIRE development group. Publication guidelines for quality improvement studies in health care: evolution of the SQUIRE project. BMJ 2009;338:a3152.
20. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al; CHEERS Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346:f1049.
21. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 2010;1:94-9.
22. RIGHT Statement. About RIGHT. 2014. Disponibile a: www.right-statement.org. Ultimo accesso: 23 febbraio 2017.
23. Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2:1-88.
24. Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med 2011;8:e1000393.
25. Greenland S, O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics 2001;2:463-71.
26. Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282:1054-60.
27. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al; AGREE Next Steps Consortium. The Global Rating Scale complements the AGREE II in advancing the quality of practice guidelines. J Clin Epidemiol 2012;65:526-34.
28. Grimmer K, Dizon JM, Milanese S, King E, Beaton K, Thorpe O, et al. Efficient clinical evaluation of guideline quality: development and testing of a new tool. BMC Med Res Methodol 2014;14:63.
29. Schünemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ 2014;186:E123-42.
30. Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 2014;383:267-76.
31. Ansari S, Rashidian A. Guidelines for guidelines: are they up to the task? A comparative assessment of clinical practice guideline development handbooks. PLoS One. 2012;7:e49864. [PMID:23189167] doi:10.1371/journal.pone.0049864
32. Vernooij RW, Sanabria AJ, Solà I, Alonso-Coello P, Martínez García L. Guidance for updating clinical practice guidelines: a systematic review of methodological handbooks. Implement Sci. 2014;9:3. [PMID: 24383701] doi:10.1186/1748-5908-9-3