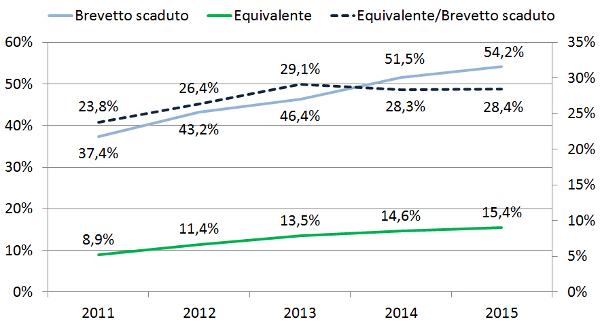
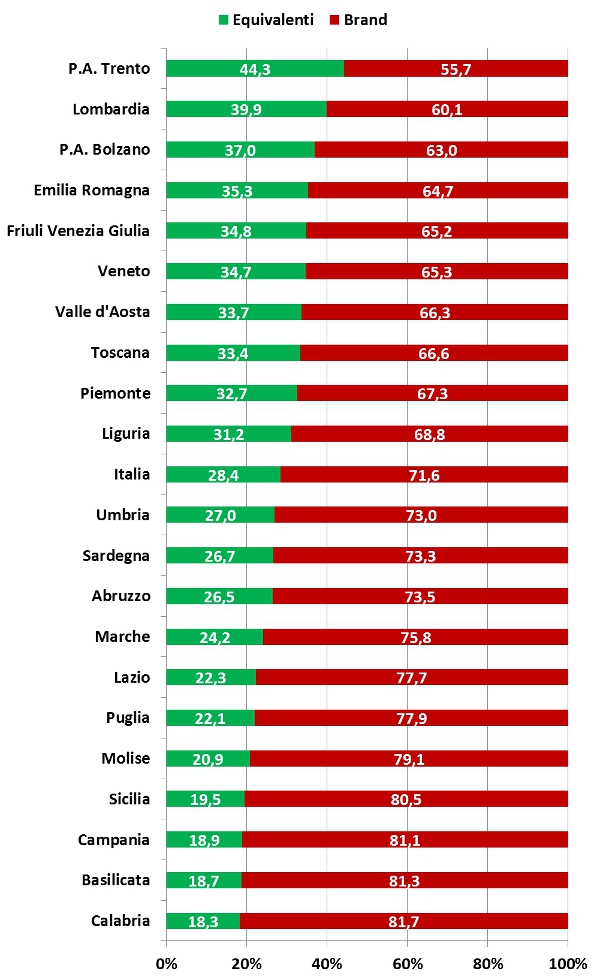
Position Statement GIMBE
Il sotto-utilizzo dei farmaci equivalenti in Italia
Antonino Cartabellotta, Corrado IaconoEvidence 2016;8(10): e1000153 doi: 10.4470/E1000153
Pubblicato: 21 ottobre 2016
Copyright: © 2016 Cartabellotta et al. Questo è un articolo open-access, distribuito con licenza Creative Commons Attribution, che ne consente lâutilizzo, la distribuzione e la riproduzione su qualsiasi supporto esclusivamente per fini non commerciali, a condizione di riportare sempre autore e citazione originale.
1. Rapporto sulla sostenibilità del Servizio Sanitario Nazionale 2016-2025. Fondazione GIMBE: Bologna, giugno 2016. Disponibile a: www.rapportogimbe.it. Ultimo accesso: 21 ottobre 2016.
2. Cartabellotta A. #salviamoSSN: dal Rapporto GIMBE allâOsservatorio sulla sostenibilità del Servizio Sanitario Nazionale. Evidence 2016;8(9): e1000151.
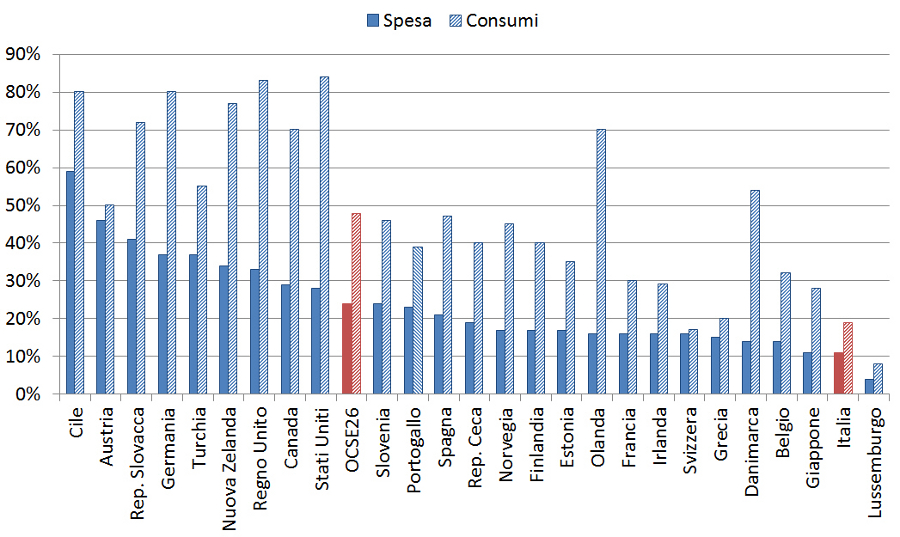
3. OECD. Uno sguardo sulla sanità 2015: come si posiziona lâItalia. Disponibile a: www.oecd.org/italy/Health-at-a-Glance-2015-Key-Findings-ITALY-In-Italian.pdf. Ultimo accesso: 21 ottobre 2016.
4. Agenzia Italiana del Farmaco. Liste di trasparenza e rimborsabilità . Disponibile a: www.agenziafarmaco.gov.it/it/content/liste-ditrasparenza-e-rimborsabilit%C3%A0. Ultimo accesso: 21 ottobre 2016.
5. Art. 15 c. 11 bis D.L. 95/2012 convertito in L. 135/2012
6. Art. 11 c. 12 D.L. 1/2012 convertito in L. 27/2012
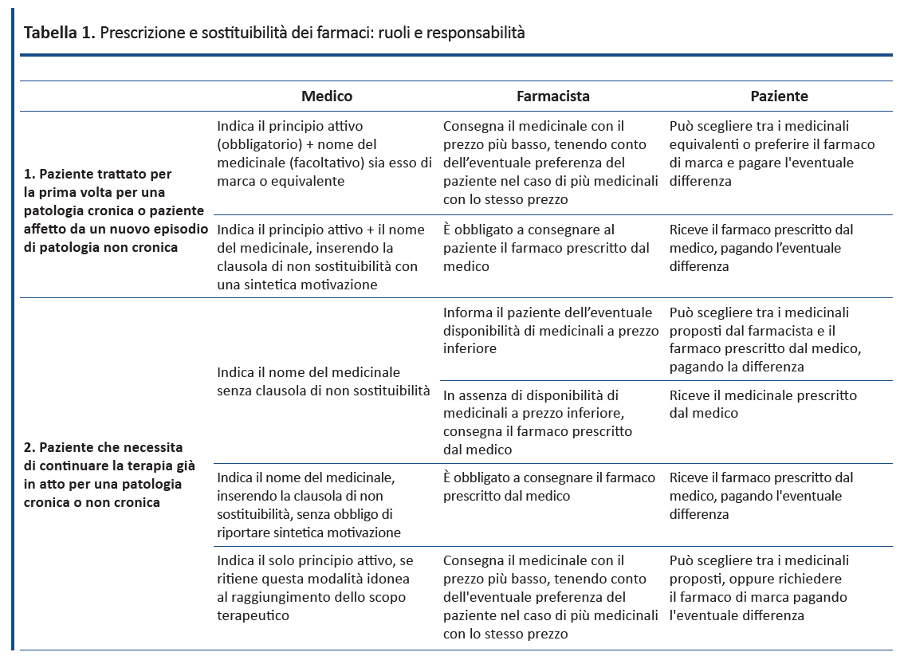
7. Ministero della Salute. Nuove ricette: cosa devono fare medici e farmacisti. Aggiornamento al 9 gennaio 2013. Disponibile a: www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=dalministero&id=456. Ultimo accesso 21 ottobre 2016.
8. Parere dellâUfficio Legislativo del Ministero della Salute del 15/04/2015. Disponibile a: www.salute.gov.it/imgs/C_17_pubblicazioni_2355_allegato.pdf. Ultimo accesso 21 ottobre 2016.
9. Agenzia Italiana del Farmaco. Medicinali Equivalenti: qualità , sicurezza ed efficacia. Roma, dicembre 2015. Disponibile a: www.agenziafarmaco.gov.it/sites/default/files/medicinali_equivalenti-qualita_sicurezza_efficacia.pdf. Ultimo accesso: 21 ottobre 2016
10. Agenzia Italiana del Farmaco. âEquivalenti o generici: quello che i pazienti devono sapereâ. Statement e documento di domande e risposte. Roma, settembre 2012. Disponibile a: www.agenziafarmaco.gov.it/it/content/%E2%80%9Cequivalenti-o-generici-quello-che-i-pazienti-devono-sapere%E2%80%9D-statement-e-documento-di-domand. Ultimo accesso: 21 ottobre 2016.
11. Choudhry NK, Denberg TD, Qaseem A; Clinical Guidelines Committee of American College of Physicians. Improving Adherence to Therapy and Clinical Outcomes While Containing Costs: Opportunities From the Greater Use of Generic Medications: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2016;164:41-9.
12. U.S. Department of Health and Human Services. Expanding the Use of Generic Drugs. Washington, DC: U.S. Department of Health and Human Services; 2010. Disponibile a: https://aspe.hhs.gov/basic-report/expanding-use-generic-drugs. Ultimo accesso 21 ottobre 2016.
13. Federman AD, Halm EA, Siu AL. Use of generic cardiovascular medications by elderly Medicare beneficiaries receiving generalist or cardiologist care. Med Care. 2007;45:109-15.
14. Gellad WF, Donohue JM, Zhao X, Mor MK, Thorpe CT, Smith J, et al. Brand-name prescription drug use among Veterans Affairs and Medicare Part D patients with diabetes: a national cohort comparison. Ann Intern Med. 2013;159:105-14.
15. Mott DA, Cline RR. Exploring generic drug use behavior: the role of prescribers and pharmacists in the opportunity for generic drug use and generic substitution. Med Care 2002;40:662-74.
16. Haas JS, Phillips KA, Gerstenberger EP, Seger AC. Potential savings from substituting generic drugs for brand-name drugs: Medical Expenditure Panel Survey, 1997â2000. Ann Intern Med 2005;142: 891-7.
17. Choudhry NK, Levin R, Avorn J. The economic consequences of non-evidence-based clopidogrel use. Am Heart J. 2008;155:904-9.
18. Fischer MA, Avorn J. Economic implications of evidence-based prescribing for hypertension: can better care cost less? JAMA 2004; 291:1850-6.
19. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206-52.
20. Desai NR, Shrank WH, Fischer MA, Avorn J, Liberman JN, Schneeweiss S, et al. Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications. Am J Med. 2012;125:302.e1-7.
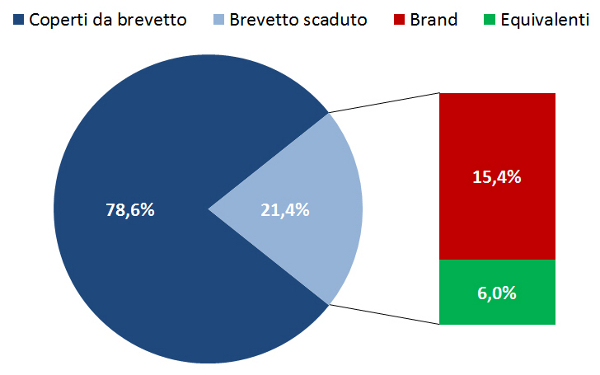
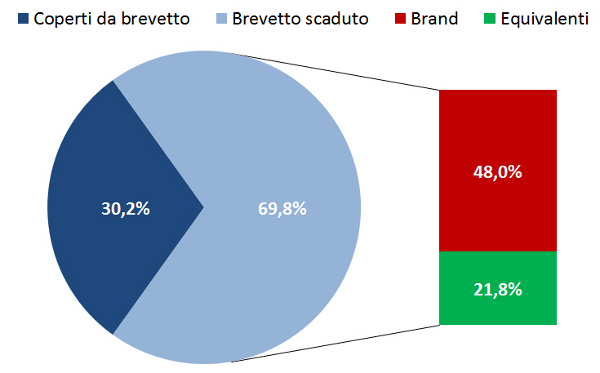
21. Osservatorio Nazionale sullâimpiego dei Medicinali. Lâuso dei farmaci in Italia. Rapporto Nazionale 2015. Roma: Agenzia Italiana del Farmaco, giugno 2016. Disponibile a: www.agenziafarmaco.gov.it/it/content/luso-dei-farmaci-italia-rapporto-osmed-2015. Ultimo accesso: 21 ottobre 2016.
22. Agenzia Italiana del Farmaco. Monitoraggio della Spesa Farmaceutica Nazionale e Regionale Gennaio-Maggio 2016. Riunione CdA del 15 settembre 2016. Disponibile a: www.agenziafarmaco.gov.it/sites/default/files/Estratto_Monitoraggio_della_Spesa_gen-mag_2016.pdf. Ultimo accesso: 21 ottobre 2016.
23. Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298:61-9.
24. Shrank WH, Choudhry NK, Fischer MA, Avorn J, Powell M, Schneeweiss S, et al. The epidemiology of prescriptions abandoned at the pharmacy. Ann Intern Med. 2010;153:633-40.
25. Roebuck MC, Liberman JN, Gemmill-Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff (Millwood). 2011;30:91-9.
26. Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care 2005;43:521-30.
27. Stuart BC, Simoni-Wastila L, Zhao L, Lloyd JT, Doshi JA. Increased persistency in medication use by U.S. Medicare beneficiaries with diabetes is associated with lower hospitalization rates and cost savings. Diabetes Care 2009;32:647-9.
28. Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Medication adherence and use of generic drug therapies. Am J Manag Care 2009;15:450-6.
29. Shrank WH, Hoang T, Ettner SL, Glassman PA, Nair K, DeLapp D, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med 2006;166:332-7.
30. Taira DA, Wong KS, Frech-Tamas F, Chung RS. Copayment level and compliance with antihypertensive medication: analysis and policy implications for managed care. Am J Manag Care 2006;12:678- 83.
31. Sedjo RL, Cox ER. Lowering copayments: impact of simvastatin patent expiration on patient adherence. Am J Manag Care 2008;14: 813-8.
32. Gagne JJ, Choudhry NK, Kesselheim AS, Polinski JM, Hutchins D, Matlin OS, et al. Comparative effectiveness of generic and brandname statins on patient outcomes: a cohort study. Ann Intern Med 2014;161:400-7.
33. Rosenthal E. Officials question the rising costs of generic drugs. The New York Times. 8 October 2014:B9.
34. Montebelli MR. Usa. La guerra dei generici, tra aumenti iperbolici di prezzo e offerte da discount. Quotidiano Sanità , 28 ottobre 2015. Disponibile a: www.quotidianosanita.it/scienza-e-farmaci/articolo.php?articolo_id=32751. Ultimo accesso: 21 ottobre 2016.
35. Strom BL. Generic drug substitution revisited. N Engl J Med 1987;316:1456-62.
36. Kesselheim AS, Misono AS, Lee JL, Stedman MR, Brookhart MA, Choudhry NK, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA 2008;300:2514-26.
37. Flacco ME, Manzoli L, Boccia S, Puggina A, Rosso A, Marzuillo C, et al .Registered randomized trials comparing generic and brand-name drugs: a survey. Mayo Clin Proc 2016;91:1021-34.
38. Dentali F, Donadini MP, Clark N, Crowther MA, Garcia D, Hylek E, et al; Warfarin Associated Research Projects and Other Endeavors (WARPED) Consortium. Brand name versus generic warfarin: a systematic review of the literature. Pharmacotherapy 2011;31:386-93.
39. Manzoli L, Flacco ME, Boccia S, DâAndrea E, Panic N, Marzuillo C, et al. Generic versus brand-name drugs used in cardiovascular diseases. Eur J Epidemiol. 2016;31:351-68.
40. Aronica A, Colombo GL, Di Matteo S, Visconti M, gruppo di ricerca MySearch, Co.S - Consorzio Sanità . Il farmaco equivalente nella pratica clinica. I risultati di una survey in area cardiovascolare presso cooperative di Medici di Medicina Generale. Clinico Economics 2011 6:15-26.
41. Colombo GL, Agabiti-Rosei E, Margonato A, Mencacci C, Montecucco CM, Trevisan R. Off-patent generic medicines vs. off-patent brand medicines for six reference drugs: a retrospective claims data study from five local healthcare units in the Lombardy Region of Italy. PLoS One 2013;8(12):e82990.
42. Ip S, Chung M, Moorthy D, et al. Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease: Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 Sep. (Comparative Effectiveness Reviews, No. 29.). Disponibile a: https://effectivehealthcare.ahrq.gov/ehc/products/165/755/CER29-GERD_20110926.pdf. Ultimo accesso: 21 ottobre 2016.
43. McDonagh MS, Carson S, Thakurta S. Drug Class Review: Proton Pump Inhibitors: Final Report Update 5. Portland: Oregon Health & Science University; 2009.
44. Mottram P, Wilson K, Strobl J. Antidepressants for depressed elderly. Cochrane Database Syst Rev. 2006:CD003491.
45. Gartlehner G, Hansen RA, Kahwati L, Lohr KN, Gaynes B, Carey T. Drug Class Review on Second Generation Antidepressants: Final Report. Portland: Oregon Health & Science University; 2006.
46. Qaseem A, Snow V, Denberg TD, Forciea MA, Owens DK; Clinical Efficacy Assessment Subcommittee of American College of Physicians. Using second-generation antidepressants to treat depressive disorders: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2008;149:725-33.
47. Kesselheim AS, Stedman MR, Bubrick EJ, Gagne JJ, Misono AS, Lee JL, et al. Seizure outcomes following the use of generic versus brand-name antiepileptic drugs: a systematic review and metaanalysis. Drugs 2010;70:605-21.
48. Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, et al; Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI FREEE) Trial. Full coverage for preventive medications after myocardial infarction. N Engl J Med 2011;365:2088- 97.
49. Berkowitz SA, Krumme AA, Avorn J, Brennan T, Matlin OS, Spettell CM, et al. Initial choice of oral glucose-lowering medication for diabetes mellitus: a patient-centered comparative effectiveness study. JAMA Intern Med 2014;174:1955-62.
50. Grant RW, Pabon-Nau L, Ross KM, Youatt EJ, Pandiscio JC, Park ER. Diabetes oral medication initiation and intensification: patient views compared with current treatment guidelines. Diabetes Educ 2011;37:78-84.
51. Huang ES, Brown SE, Ewigman BG, Foley EC, Meltzer DO. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care 2007;30:2478-83.
52. Stewart WC, Sharpe ED, Stewart JA, Hott CE. The safety and efficacy of timolol 0.5% in xanthan gum versus timolol gel forming solution 0.5%. Curr Eye Res. 2002;24:387-91.
53. Narayanaswamy A, Neog A, Baskaran M, George R, Lingam V, Desai C, et al. A randomized, crossover, open label pilot study to evaluate the efficacy and safety of Xalatan in comparison with generic latanoprost (Latoprost) in subjects with primary open angle glaucoma or ocular hypertension. Indian J Ophthalmol. 2007;55: 127-31.
54. Hashemi M, Miraftabi A, Nilforoushan N, Ghasemi Falavarjani K, Pakdel F, Soudi R, et al. Comparison of the efficacy and tolerability of Xalatan® and Xalabiost (generic latanoprost) in adults with openangle glaucoma or ocular hypertension: a two-center, randomized, crossover trial. Iran J Ophthalmol 2012;24:11-8.
55. Golan S, Rosenfeld E, Shemesh G, Kurtz S. Original and generic latanoprost for the treatment of glaucoma and ocular hypertension: are they really the same? Clin Exp Pharmacol Physiol. 2015;42:220-4.
56. Digiuni M, Manni G, Vetrugno M, Uva M, Milano G, Orzalesi N, et al. An evaluation of therapeutic noninferiority of 0.005% latanoprost ophthalmic solution and Xalatan in patients with glaucoma or ocular hypertension. J Glaucoma. 2013;22:707-12.
57. Brennan TA, Lee TH. Allergic to generics. Ann Intern Med 2004; 141:126-30.
58. Shrank WH, Cox ER, Fischer MA, Mehta J, Choudhry NK. Patientsâ perceptions of generic medications. Health Aff (Millwood) 2009;28:546-56.
59. Shrank WH, Liberman JN, Fischer MA, Girdish C, Brennan TA, Choudhry NK. Physician perceptions about generic drugs. Ann Pharmacother 2011;45:31-8.
60. Frisk P, Rydberg T, Carlsten A, Ekedahl A. Patientsâ experiences with generic substitution: a Swedish pharmacy survey. J Pharm Health Serv Res 2011;2:9-15.
61. Waber RL, Shiv B, Carmon Z, Ariely D. Commercial features of placebo and therapeutic efficacy. JAMA 2008;299:1016-7.
62. Kesselheim AS, Bykov K, Avorn J, Tong A, Doherty M, Choudhry NK. Burden of changes in pill appearance for patients receiving generic cardiovascular medications after myocardial infarction: cohort and nested caseâcontrol studies. Ann Intern Med 2014;161:96-103.
63. Kesselheim AS, Misono AS, Shrank WH, Greene JA, Doherty M, Avorn J, et al. Variations in pill appearance of antiepileptic drugs and the risk of nonadherence. JAMA Intern Med 2013;173:202-8.
64. Banahan BF 3rd, Kolassa EM. A physician survey on generic drugs and substitution of critical dose medications. Arch Intern Med 1997;157:2080-8.
65. Wang B, Gagne JJ, Choudhry NK. The epidemiology of drug recalls in the United States. Arch Intern Med. 2012;172:1109-10.
66. Helfand C. Top 10 generics makers by 2012 revenue. Fierce Pharma. 21 October 2013.
67. Steinman MA, Chren MM, Landefeld CS. Whatâs in a name? Use of brand versus generic drug names in United States outpatient practice. J Gen Intern Med. 2007;22:645-8.
68. Shrank WH, Liberman JN, Fischer MA, Avorn J, Kilabuk E, Chang A, et al. The consequences of requesting âdispense as writtenâ. Am J Med. 2011;124:309-17.
69. Campbell EG, Pham-Kanter G, Vogeli C, Iezzoni LI. Physician acquiescence to patient demands for brand-name drugs: results of a national survey of physicians. JAMA Intern Med 2013;173: 237-9.
70. Campbell EG. Doctors and drug companiesâscrutinizing influential relationships. N Engl J Med 2007;357:1796-7.
71. Nomisma. Il sistema dei farmaci generici in Italia. Scenari per una crescita sostenibile. Bologna: maggio 2015. Disponibile a: https://www.senato.it/application/xmanager/projects/leg17/attachments/documento_evento_procedura_commissione/files/000/002/688/STUDIO_NOMISMA_2.pdf. Ultimo accesso: 21 ottobre 2016.
72. Shrank WH, Choudhry NK, Agnew-Blais J, Federman AD, Liberman JN, Liu J, et al. State generic substitution laws can lower drug outlays under Medicaid. Health Aff (Millwood) 2010;29:1383-90.
73. Fischer MA, Vogeli C, Stedman M, Ferris T, Brookhart MA, Weissman JS. Effect of electronic prescribing with formulary decision support on medication use and cost. Arch Intern Med 2008;168:2433-9.
74. Stenner SP, Chen Q, Johnson KB. Impact of generic substitution decision support on electronic prescribing behavior. J Am Med Inform Assoc 2010;17:681-8.
75. Johnson MJ, May CR. Promoting professional behaviour change in healthcare: what interventions work, and why? A theory-led overview of systematic reviews BMJ Open 2015;5:e008592.
76. Forsetlund L, Bjørndal A, Rashidian A, Jamtvedt G, OâBrien MA, Wolf F, Davis D, Odgaard-Jensen J, Oxman AD. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2009;(2):CD003030.
77. OâBrien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard-Jensen J, Kristoffersen DT, Forsetlund L, Bainbridge D, Freemantle N, Davis D, Haynes R.B, Harvey E. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No.: CD000409
78. Agenzia Sanitaria e Sociale Emilia Romagna. Programma farmacista facilitatore e audit & feedback. Disponibile a: http://assr.regione.emilia-romagna.it/it/aree_attivita/valutazione-del-farmaco/informazione-indipendente/programma_farmacista_facilitatore. Ultimo accesso: 21 ottobre 2016.
79. Petrucci P, Peluso E. Lâattività del farmacista: facilitatore per i medici di medicina generale per il raggiungimento degli obiettivi regionali. Boll SIFO 2011;57:252-254
80. Shrank WH, Choudhry NK, Solomon DH, Snedden TM, Lee TH, Glynn RJ, et al. Rationale and design of the Study Assessing the Effect of Cardiovascular Medications Provided as Low-cost, Evidence-based Generic Samples (SAMPLES) trial. Am Heart J 2009;157:613-9.
81. Scott AB, Culley EJ, OâDonnell J. Effects of a physician office generic drug sampling system on generic dispensing ratios and drug costs in a large managed care organization. J Manag Care Pharm. 2007;13:412-9.
82. Rathe J, Larsen P, Andersen M, Paulsen M, Jarbøl D, Thomsen J, et al. Associations between generic substitution and patientsâ attitudes, beliefs and experiences. Eur J Clin Pharmacol 2013;69:1827-36.
83. OâMalley AJ, Frank RG, Kaddis A, Rothenberg BM, McNeil BJ. Impact of alternative interventions on changes in generic dispensing rates. Health Serv Res 2006;41:1876-94.
84. Cittadinanzattiva. Io Equivalgo. Disponibile a: www.ioequivalgo.it. Ultimo accesso: 21 ottobre 2016.
85. Altroconsumo. Farmaci, confronta i prezzi e trova il meno caro. Disponibile a: www.altroconsumo.it/salute/farmaci/calcola-risparmia/banca-dati-farmaci. Ultimo accesso: 21 ottobre 2016.
86. Huskamp HA, Deverka PA, Epstein AM, Epstein RS, McGuigan KA, Frank RG. The effect of incentive-based formularies on prescription-drug utilization and spending. N Engl J Med 2003;349: 2224-32.
87. Rector TS, Finch MD, Danzon PM, Pauly MV, Manda BS. Effect of tiered prescription copayments on the use of preferred brand medications. Med Care. 2003;41:398-406.
88. Kelton CM, Chang LV, Kreling DH. State Medicaid programs missed $220 million in uncaptured savings as generic fluoxetine came to market, 2001â05. Health Aff (Millwood) 2013;32:1204-11.
89. Fischer MA, Avorn J. Economic consequences of underuse of generic drugs: evidence from Medicaid and implications for prescription drug benefit plans. Health Serv Res 2003;38:1051-63.
90. Holmes DR Jr, Becker JA, Granger CB, Limacher MC, Page RL 2nd, Sila C. ACCF/AHA 2011 health policy statement on therapeutic interchange and substitution: a report of the American College of Cardiology Foundation Clinical Quality Committee. Circulation 2011;124:1290-310.
91. Sanfélix-Gimeno G, Franklin JM, Shrank WH, Carlo M, Tong AY, Reisman L, et al. Did HEDIS get it right? Evaluating the quality of a quality measure: adherence to Ã-blockers and cardiovascular outcomes after myocardial infarction. Med Care 2014;52:669-76.
92. Centers for Medicare & Medicaid Services. Five-Star Quality Rating System. Baltimore: Centers for Medicare & Medicaid Services; 2015. Disponibile a: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/FSQRS.html. Ultimo accesso: 21 ottobre 2016.
93. Doran T, Fullwood C, Gravelle H, Reeves D, Kontopantelis E, Hiroeh U, et al. Pay-for-performance programs in family practices in the United Kingdom. N Engl J Med 2006;355:375-84.
94. Lindenauer PK, Remus D, Roman S, Rothberg MB, Benjamin EM, Ma A, et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med 2007;356:486-96.
95. Petersen LA, Woodard LD, Urech T, Daw C, Sookanan S. Does pay-for-performance improve the quality of health care? Ann Intern Med 2006;145:265-72.
96. Maragò E. Governance farmaceutica. La rivoluzione delle Regioni in nove mosse. Quotidiano Sanità , 5 maggio 2016. Disponibile a: www.quotidianosanita.it/regioni-e-asl/articolo.php?articolo_id=39364. Ultimo accesso: 21 ottobre 2016.
97. Zanchetta G, Salvador A, Bozzini L, Font M, Mezzalira L. Lâuso dei generici e la lista di trasparenza. Infofarma 2016 n°3-4:2-6. Disponibile a: www.ulss20.verona.it/data/29/Informazioni/2_Forum_clinico_LISTA_DI%20TRASPARENZA-revAS-revMF.pdf. Ultimo accesso: 21 ottobre 2016.
98. Agenzia Italiana del Farmaco. Determina n. 1525/2015 del 24 novembre 2015. Procedura di pay-back (articolo 9-ter , commi 10, lettera b) e 11 del decreto-legge n. 78/2015, convertito con modificazioni dalla legge n. 125/2015) - Anni 2015-2016-2017. GU n.282 del 03.12.2015.
99. Salvador A, Bozzini L, Font M. Farmaci meno costosi per chi? gli effetti della determina AIFA sul prezzo dei medicinali. Info Farma 2015;n°6: 18-21.
100. Easthall C, Song F, Bhattacharya D. A meta-analysis of cognitive based behaviour change techniques as interventions to improve medication adherence. BMJ Open. 2013;3.
101. Stacey D, Légaré F, Col NF, Bennett CL, Barry MJ, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431.
102. Choudhry NK, Cox ER, Fischer MA, Mehta J, Shrank WH. Setting prices for generic medications: a survey of patientsâ perceptions. Am J Pharm Benefits 2010;2:33-8.
103. Sarpatwari A, Kesselheim AS. The case for reforming drug naming: should brand name trademark protections expire upon generic entry? PLoS Med 2016 ;13:e1001955.
2. Cartabellotta A. #salviamoSSN: dal Rapporto GIMBE allâOsservatorio sulla sostenibilità del Servizio Sanitario Nazionale. Evidence 2016;8(9): e1000151.
3. OECD. Uno sguardo sulla sanità 2015: come si posiziona lâItalia. Disponibile a: www.oecd.org/italy/Health-at-a-Glance-2015-Key-Findings-ITALY-In-Italian.pdf. Ultimo accesso: 21 ottobre 2016.
4. Agenzia Italiana del Farmaco. Liste di trasparenza e rimborsabilità . Disponibile a: www.agenziafarmaco.gov.it/it/content/liste-ditrasparenza-e-rimborsabilit%C3%A0. Ultimo accesso: 21 ottobre 2016.
5. Art. 15 c. 11 bis D.L. 95/2012 convertito in L. 135/2012
6. Art. 11 c. 12 D.L. 1/2012 convertito in L. 27/2012
7. Ministero della Salute. Nuove ricette: cosa devono fare medici e farmacisti. Aggiornamento al 9 gennaio 2013. Disponibile a: www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=dalministero&id=456. Ultimo accesso 21 ottobre 2016.
8. Parere dellâUfficio Legislativo del Ministero della Salute del 15/04/2015. Disponibile a: www.salute.gov.it/imgs/C_17_pubblicazioni_2355_allegato.pdf. Ultimo accesso 21 ottobre 2016.
9. Agenzia Italiana del Farmaco. Medicinali Equivalenti: qualità , sicurezza ed efficacia. Roma, dicembre 2015. Disponibile a: www.agenziafarmaco.gov.it/sites/default/files/medicinali_equivalenti-qualita_sicurezza_efficacia.pdf. Ultimo accesso: 21 ottobre 2016
10. Agenzia Italiana del Farmaco. âEquivalenti o generici: quello che i pazienti devono sapereâ. Statement e documento di domande e risposte. Roma, settembre 2012. Disponibile a: www.agenziafarmaco.gov.it/it/content/%E2%80%9Cequivalenti-o-generici-quello-che-i-pazienti-devono-sapere%E2%80%9D-statement-e-documento-di-domand. Ultimo accesso: 21 ottobre 2016.
11. Choudhry NK, Denberg TD, Qaseem A; Clinical Guidelines Committee of American College of Physicians. Improving Adherence to Therapy and Clinical Outcomes While Containing Costs: Opportunities From the Greater Use of Generic Medications: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2016;164:41-9.
12. U.S. Department of Health and Human Services. Expanding the Use of Generic Drugs. Washington, DC: U.S. Department of Health and Human Services; 2010. Disponibile a: https://aspe.hhs.gov/basic-report/expanding-use-generic-drugs. Ultimo accesso 21 ottobre 2016.
13. Federman AD, Halm EA, Siu AL. Use of generic cardiovascular medications by elderly Medicare beneficiaries receiving generalist or cardiologist care. Med Care. 2007;45:109-15.
14. Gellad WF, Donohue JM, Zhao X, Mor MK, Thorpe CT, Smith J, et al. Brand-name prescription drug use among Veterans Affairs and Medicare Part D patients with diabetes: a national cohort comparison. Ann Intern Med. 2013;159:105-14.
15. Mott DA, Cline RR. Exploring generic drug use behavior: the role of prescribers and pharmacists in the opportunity for generic drug use and generic substitution. Med Care 2002;40:662-74.
16. Haas JS, Phillips KA, Gerstenberger EP, Seger AC. Potential savings from substituting generic drugs for brand-name drugs: Medical Expenditure Panel Survey, 1997â2000. Ann Intern Med 2005;142: 891-7.
17. Choudhry NK, Levin R, Avorn J. The economic consequences of non-evidence-based clopidogrel use. Am Heart J. 2008;155:904-9.
18. Fischer MA, Avorn J. Economic implications of evidence-based prescribing for hypertension: can better care cost less? JAMA 2004; 291:1850-6.
19. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206-52.
20. Desai NR, Shrank WH, Fischer MA, Avorn J, Liberman JN, Schneeweiss S, et al. Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications. Am J Med. 2012;125:302.e1-7.
21. Osservatorio Nazionale sullâimpiego dei Medicinali. Lâuso dei farmaci in Italia. Rapporto Nazionale 2015. Roma: Agenzia Italiana del Farmaco, giugno 2016. Disponibile a: www.agenziafarmaco.gov.it/it/content/luso-dei-farmaci-italia-rapporto-osmed-2015. Ultimo accesso: 21 ottobre 2016.
22. Agenzia Italiana del Farmaco. Monitoraggio della Spesa Farmaceutica Nazionale e Regionale Gennaio-Maggio 2016. Riunione CdA del 15 settembre 2016. Disponibile a: www.agenziafarmaco.gov.it/sites/default/files/Estratto_Monitoraggio_della_Spesa_gen-mag_2016.pdf. Ultimo accesso: 21 ottobre 2016.
23. Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298:61-9.
24. Shrank WH, Choudhry NK, Fischer MA, Avorn J, Powell M, Schneeweiss S, et al. The epidemiology of prescriptions abandoned at the pharmacy. Ann Intern Med. 2010;153:633-40.
25. Roebuck MC, Liberman JN, Gemmill-Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff (Millwood). 2011;30:91-9.
26. Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care 2005;43:521-30.
27. Stuart BC, Simoni-Wastila L, Zhao L, Lloyd JT, Doshi JA. Increased persistency in medication use by U.S. Medicare beneficiaries with diabetes is associated with lower hospitalization rates and cost savings. Diabetes Care 2009;32:647-9.
28. Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Medication adherence and use of generic drug therapies. Am J Manag Care 2009;15:450-6.
29. Shrank WH, Hoang T, Ettner SL, Glassman PA, Nair K, DeLapp D, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med 2006;166:332-7.
30. Taira DA, Wong KS, Frech-Tamas F, Chung RS. Copayment level and compliance with antihypertensive medication: analysis and policy implications for managed care. Am J Manag Care 2006;12:678- 83.
31. Sedjo RL, Cox ER. Lowering copayments: impact of simvastatin patent expiration on patient adherence. Am J Manag Care 2008;14: 813-8.
32. Gagne JJ, Choudhry NK, Kesselheim AS, Polinski JM, Hutchins D, Matlin OS, et al. Comparative effectiveness of generic and brandname statins on patient outcomes: a cohort study. Ann Intern Med 2014;161:400-7.
33. Rosenthal E. Officials question the rising costs of generic drugs. The New York Times. 8 October 2014:B9.
34. Montebelli MR. Usa. La guerra dei generici, tra aumenti iperbolici di prezzo e offerte da discount. Quotidiano Sanità , 28 ottobre 2015. Disponibile a: www.quotidianosanita.it/scienza-e-farmaci/articolo.php?articolo_id=32751. Ultimo accesso: 21 ottobre 2016.
35. Strom BL. Generic drug substitution revisited. N Engl J Med 1987;316:1456-62.
36. Kesselheim AS, Misono AS, Lee JL, Stedman MR, Brookhart MA, Choudhry NK, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA 2008;300:2514-26.
37. Flacco ME, Manzoli L, Boccia S, Puggina A, Rosso A, Marzuillo C, et al .Registered randomized trials comparing generic and brand-name drugs: a survey. Mayo Clin Proc 2016;91:1021-34.
38. Dentali F, Donadini MP, Clark N, Crowther MA, Garcia D, Hylek E, et al; Warfarin Associated Research Projects and Other Endeavors (WARPED) Consortium. Brand name versus generic warfarin: a systematic review of the literature. Pharmacotherapy 2011;31:386-93.
39. Manzoli L, Flacco ME, Boccia S, DâAndrea E, Panic N, Marzuillo C, et al. Generic versus brand-name drugs used in cardiovascular diseases. Eur J Epidemiol. 2016;31:351-68.
40. Aronica A, Colombo GL, Di Matteo S, Visconti M, gruppo di ricerca MySearch, Co.S - Consorzio Sanità . Il farmaco equivalente nella pratica clinica. I risultati di una survey in area cardiovascolare presso cooperative di Medici di Medicina Generale. Clinico Economics 2011 6:15-26.
41. Colombo GL, Agabiti-Rosei E, Margonato A, Mencacci C, Montecucco CM, Trevisan R. Off-patent generic medicines vs. off-patent brand medicines for six reference drugs: a retrospective claims data study from five local healthcare units in the Lombardy Region of Italy. PLoS One 2013;8(12):e82990.
42. Ip S, Chung M, Moorthy D, et al. Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease: Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 Sep. (Comparative Effectiveness Reviews, No. 29.). Disponibile a: https://effectivehealthcare.ahrq.gov/ehc/products/165/755/CER29-GERD_20110926.pdf. Ultimo accesso: 21 ottobre 2016.
43. McDonagh MS, Carson S, Thakurta S. Drug Class Review: Proton Pump Inhibitors: Final Report Update 5. Portland: Oregon Health & Science University; 2009.
44. Mottram P, Wilson K, Strobl J. Antidepressants for depressed elderly. Cochrane Database Syst Rev. 2006:CD003491.
45. Gartlehner G, Hansen RA, Kahwati L, Lohr KN, Gaynes B, Carey T. Drug Class Review on Second Generation Antidepressants: Final Report. Portland: Oregon Health & Science University; 2006.
46. Qaseem A, Snow V, Denberg TD, Forciea MA, Owens DK; Clinical Efficacy Assessment Subcommittee of American College of Physicians. Using second-generation antidepressants to treat depressive disorders: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2008;149:725-33.
47. Kesselheim AS, Stedman MR, Bubrick EJ, Gagne JJ, Misono AS, Lee JL, et al. Seizure outcomes following the use of generic versus brand-name antiepileptic drugs: a systematic review and metaanalysis. Drugs 2010;70:605-21.
48. Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, et al; Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI FREEE) Trial. Full coverage for preventive medications after myocardial infarction. N Engl J Med 2011;365:2088- 97.
49. Berkowitz SA, Krumme AA, Avorn J, Brennan T, Matlin OS, Spettell CM, et al. Initial choice of oral glucose-lowering medication for diabetes mellitus: a patient-centered comparative effectiveness study. JAMA Intern Med 2014;174:1955-62.
50. Grant RW, Pabon-Nau L, Ross KM, Youatt EJ, Pandiscio JC, Park ER. Diabetes oral medication initiation and intensification: patient views compared with current treatment guidelines. Diabetes Educ 2011;37:78-84.
51. Huang ES, Brown SE, Ewigman BG, Foley EC, Meltzer DO. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care 2007;30:2478-83.
52. Stewart WC, Sharpe ED, Stewart JA, Hott CE. The safety and efficacy of timolol 0.5% in xanthan gum versus timolol gel forming solution 0.5%. Curr Eye Res. 2002;24:387-91.
53. Narayanaswamy A, Neog A, Baskaran M, George R, Lingam V, Desai C, et al. A randomized, crossover, open label pilot study to evaluate the efficacy and safety of Xalatan in comparison with generic latanoprost (Latoprost) in subjects with primary open angle glaucoma or ocular hypertension. Indian J Ophthalmol. 2007;55: 127-31.
54. Hashemi M, Miraftabi A, Nilforoushan N, Ghasemi Falavarjani K, Pakdel F, Soudi R, et al. Comparison of the efficacy and tolerability of Xalatan® and Xalabiost (generic latanoprost) in adults with openangle glaucoma or ocular hypertension: a two-center, randomized, crossover trial. Iran J Ophthalmol 2012;24:11-8.
55. Golan S, Rosenfeld E, Shemesh G, Kurtz S. Original and generic latanoprost for the treatment of glaucoma and ocular hypertension: are they really the same? Clin Exp Pharmacol Physiol. 2015;42:220-4.
56. Digiuni M, Manni G, Vetrugno M, Uva M, Milano G, Orzalesi N, et al. An evaluation of therapeutic noninferiority of 0.005% latanoprost ophthalmic solution and Xalatan in patients with glaucoma or ocular hypertension. J Glaucoma. 2013;22:707-12.
57. Brennan TA, Lee TH. Allergic to generics. Ann Intern Med 2004; 141:126-30.
58. Shrank WH, Cox ER, Fischer MA, Mehta J, Choudhry NK. Patientsâ perceptions of generic medications. Health Aff (Millwood) 2009;28:546-56.
59. Shrank WH, Liberman JN, Fischer MA, Girdish C, Brennan TA, Choudhry NK. Physician perceptions about generic drugs. Ann Pharmacother 2011;45:31-8.
60. Frisk P, Rydberg T, Carlsten A, Ekedahl A. Patientsâ experiences with generic substitution: a Swedish pharmacy survey. J Pharm Health Serv Res 2011;2:9-15.
61. Waber RL, Shiv B, Carmon Z, Ariely D. Commercial features of placebo and therapeutic efficacy. JAMA 2008;299:1016-7.
62. Kesselheim AS, Bykov K, Avorn J, Tong A, Doherty M, Choudhry NK. Burden of changes in pill appearance for patients receiving generic cardiovascular medications after myocardial infarction: cohort and nested caseâcontrol studies. Ann Intern Med 2014;161:96-103.
63. Kesselheim AS, Misono AS, Shrank WH, Greene JA, Doherty M, Avorn J, et al. Variations in pill appearance of antiepileptic drugs and the risk of nonadherence. JAMA Intern Med 2013;173:202-8.
64. Banahan BF 3rd, Kolassa EM. A physician survey on generic drugs and substitution of critical dose medications. Arch Intern Med 1997;157:2080-8.
65. Wang B, Gagne JJ, Choudhry NK. The epidemiology of drug recalls in the United States. Arch Intern Med. 2012;172:1109-10.
66. Helfand C. Top 10 generics makers by 2012 revenue. Fierce Pharma. 21 October 2013.
67. Steinman MA, Chren MM, Landefeld CS. Whatâs in a name? Use of brand versus generic drug names in United States outpatient practice. J Gen Intern Med. 2007;22:645-8.
68. Shrank WH, Liberman JN, Fischer MA, Avorn J, Kilabuk E, Chang A, et al. The consequences of requesting âdispense as writtenâ. Am J Med. 2011;124:309-17.
69. Campbell EG, Pham-Kanter G, Vogeli C, Iezzoni LI. Physician acquiescence to patient demands for brand-name drugs: results of a national survey of physicians. JAMA Intern Med 2013;173: 237-9.
70. Campbell EG. Doctors and drug companiesâscrutinizing influential relationships. N Engl J Med 2007;357:1796-7.
71. Nomisma. Il sistema dei farmaci generici in Italia. Scenari per una crescita sostenibile. Bologna: maggio 2015. Disponibile a: https://www.senato.it/application/xmanager/projects/leg17/attachments/documento_evento_procedura_commissione/files/000/002/688/STUDIO_NOMISMA_2.pdf. Ultimo accesso: 21 ottobre 2016.
72. Shrank WH, Choudhry NK, Agnew-Blais J, Federman AD, Liberman JN, Liu J, et al. State generic substitution laws can lower drug outlays under Medicaid. Health Aff (Millwood) 2010;29:1383-90.
73. Fischer MA, Vogeli C, Stedman M, Ferris T, Brookhart MA, Weissman JS. Effect of electronic prescribing with formulary decision support on medication use and cost. Arch Intern Med 2008;168:2433-9.
74. Stenner SP, Chen Q, Johnson KB. Impact of generic substitution decision support on electronic prescribing behavior. J Am Med Inform Assoc 2010;17:681-8.
75. Johnson MJ, May CR. Promoting professional behaviour change in healthcare: what interventions work, and why? A theory-led overview of systematic reviews BMJ Open 2015;5:e008592.
76. Forsetlund L, Bjørndal A, Rashidian A, Jamtvedt G, OâBrien MA, Wolf F, Davis D, Odgaard-Jensen J, Oxman AD. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2009;(2):CD003030.
77. OâBrien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard-Jensen J, Kristoffersen DT, Forsetlund L, Bainbridge D, Freemantle N, Davis D, Haynes R.B, Harvey E. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No.: CD000409
78. Agenzia Sanitaria e Sociale Emilia Romagna. Programma farmacista facilitatore e audit & feedback. Disponibile a: http://assr.regione.emilia-romagna.it/it/aree_attivita/valutazione-del-farmaco/informazione-indipendente/programma_farmacista_facilitatore. Ultimo accesso: 21 ottobre 2016.
79. Petrucci P, Peluso E. Lâattività del farmacista: facilitatore per i medici di medicina generale per il raggiungimento degli obiettivi regionali. Boll SIFO 2011;57:252-254
80. Shrank WH, Choudhry NK, Solomon DH, Snedden TM, Lee TH, Glynn RJ, et al. Rationale and design of the Study Assessing the Effect of Cardiovascular Medications Provided as Low-cost, Evidence-based Generic Samples (SAMPLES) trial. Am Heart J 2009;157:613-9.
81. Scott AB, Culley EJ, OâDonnell J. Effects of a physician office generic drug sampling system on generic dispensing ratios and drug costs in a large managed care organization. J Manag Care Pharm. 2007;13:412-9.
82. Rathe J, Larsen P, Andersen M, Paulsen M, Jarbøl D, Thomsen J, et al. Associations between generic substitution and patientsâ attitudes, beliefs and experiences. Eur J Clin Pharmacol 2013;69:1827-36.
83. OâMalley AJ, Frank RG, Kaddis A, Rothenberg BM, McNeil BJ. Impact of alternative interventions on changes in generic dispensing rates. Health Serv Res 2006;41:1876-94.
84. Cittadinanzattiva. Io Equivalgo. Disponibile a: www.ioequivalgo.it. Ultimo accesso: 21 ottobre 2016.
85. Altroconsumo. Farmaci, confronta i prezzi e trova il meno caro. Disponibile a: www.altroconsumo.it/salute/farmaci/calcola-risparmia/banca-dati-farmaci. Ultimo accesso: 21 ottobre 2016.
86. Huskamp HA, Deverka PA, Epstein AM, Epstein RS, McGuigan KA, Frank RG. The effect of incentive-based formularies on prescription-drug utilization and spending. N Engl J Med 2003;349: 2224-32.
87. Rector TS, Finch MD, Danzon PM, Pauly MV, Manda BS. Effect of tiered prescription copayments on the use of preferred brand medications. Med Care. 2003;41:398-406.
88. Kelton CM, Chang LV, Kreling DH. State Medicaid programs missed $220 million in uncaptured savings as generic fluoxetine came to market, 2001â05. Health Aff (Millwood) 2013;32:1204-11.
89. Fischer MA, Avorn J. Economic consequences of underuse of generic drugs: evidence from Medicaid and implications for prescription drug benefit plans. Health Serv Res 2003;38:1051-63.
90. Holmes DR Jr, Becker JA, Granger CB, Limacher MC, Page RL 2nd, Sila C. ACCF/AHA 2011 health policy statement on therapeutic interchange and substitution: a report of the American College of Cardiology Foundation Clinical Quality Committee. Circulation 2011;124:1290-310.
91. Sanfélix-Gimeno G, Franklin JM, Shrank WH, Carlo M, Tong AY, Reisman L, et al. Did HEDIS get it right? Evaluating the quality of a quality measure: adherence to Ã-blockers and cardiovascular outcomes after myocardial infarction. Med Care 2014;52:669-76.
92. Centers for Medicare & Medicaid Services. Five-Star Quality Rating System. Baltimore: Centers for Medicare & Medicaid Services; 2015. Disponibile a: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/FSQRS.html. Ultimo accesso: 21 ottobre 2016.
93. Doran T, Fullwood C, Gravelle H, Reeves D, Kontopantelis E, Hiroeh U, et al. Pay-for-performance programs in family practices in the United Kingdom. N Engl J Med 2006;355:375-84.
94. Lindenauer PK, Remus D, Roman S, Rothberg MB, Benjamin EM, Ma A, et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med 2007;356:486-96.
95. Petersen LA, Woodard LD, Urech T, Daw C, Sookanan S. Does pay-for-performance improve the quality of health care? Ann Intern Med 2006;145:265-72.
96. Maragò E. Governance farmaceutica. La rivoluzione delle Regioni in nove mosse. Quotidiano Sanità , 5 maggio 2016. Disponibile a: www.quotidianosanita.it/regioni-e-asl/articolo.php?articolo_id=39364. Ultimo accesso: 21 ottobre 2016.
97. Zanchetta G, Salvador A, Bozzini L, Font M, Mezzalira L. Lâuso dei generici e la lista di trasparenza. Infofarma 2016 n°3-4:2-6. Disponibile a: www.ulss20.verona.it/data/29/Informazioni/2_Forum_clinico_LISTA_DI%20TRASPARENZA-revAS-revMF.pdf. Ultimo accesso: 21 ottobre 2016.
98. Agenzia Italiana del Farmaco. Determina n. 1525/2015 del 24 novembre 2015. Procedura di pay-back (articolo 9-ter , commi 10, lettera b) e 11 del decreto-legge n. 78/2015, convertito con modificazioni dalla legge n. 125/2015) - Anni 2015-2016-2017. GU n.282 del 03.12.2015.
99. Salvador A, Bozzini L, Font M. Farmaci meno costosi per chi? gli effetti della determina AIFA sul prezzo dei medicinali. Info Farma 2015;n°6: 18-21.
100. Easthall C, Song F, Bhattacharya D. A meta-analysis of cognitive based behaviour change techniques as interventions to improve medication adherence. BMJ Open. 2013;3.
101. Stacey D, Légaré F, Col NF, Bennett CL, Barry MJ, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431.
102. Choudhry NK, Cox ER, Fischer MA, Mehta J, Shrank WH. Setting prices for generic medications: a survey of patientsâ perceptions. Am J Pharm Benefits 2010;2:33-8.
103. Sarpatwari A, Kesselheim AS. The case for reforming drug naming: should brand name trademark protections expire upon generic entry? PLoS Med 2016 ;13:e1001955.