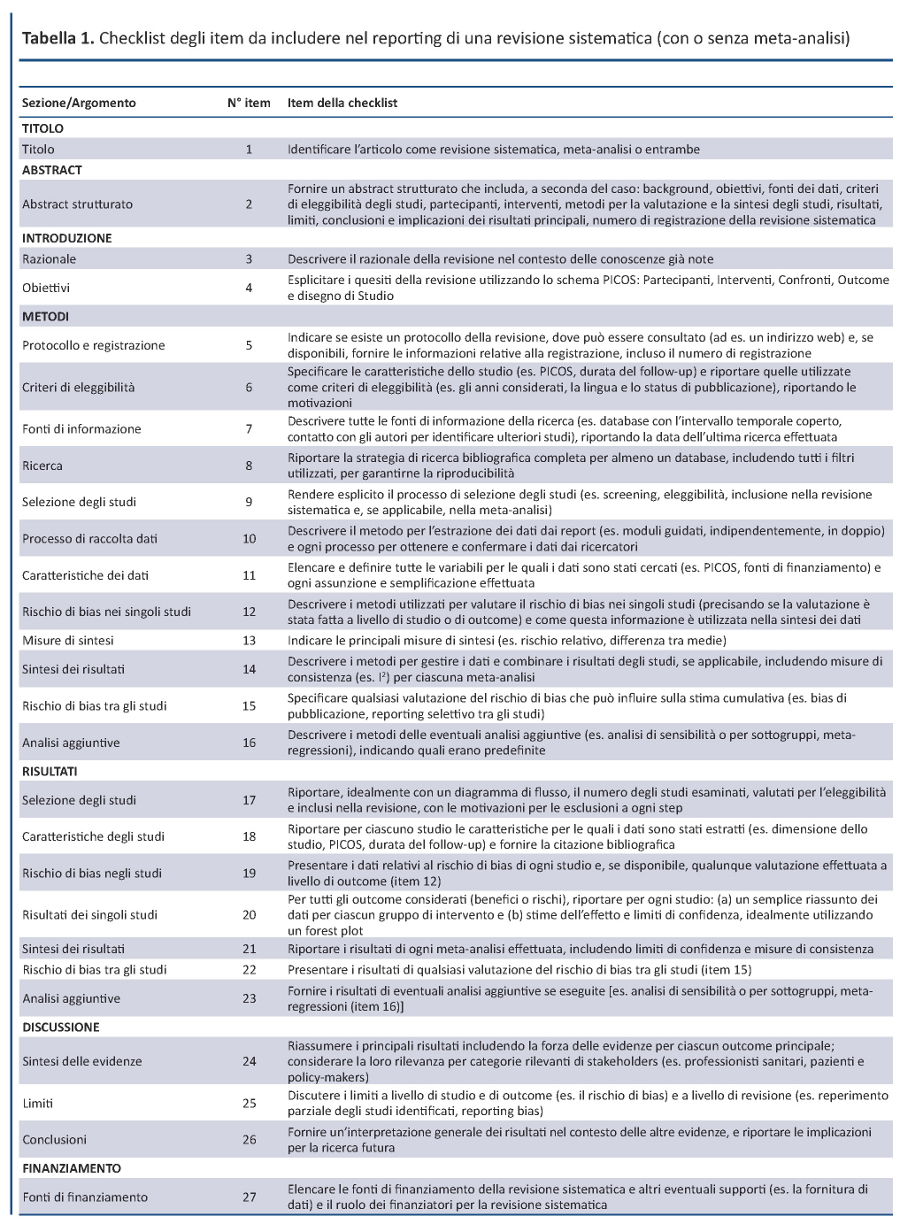
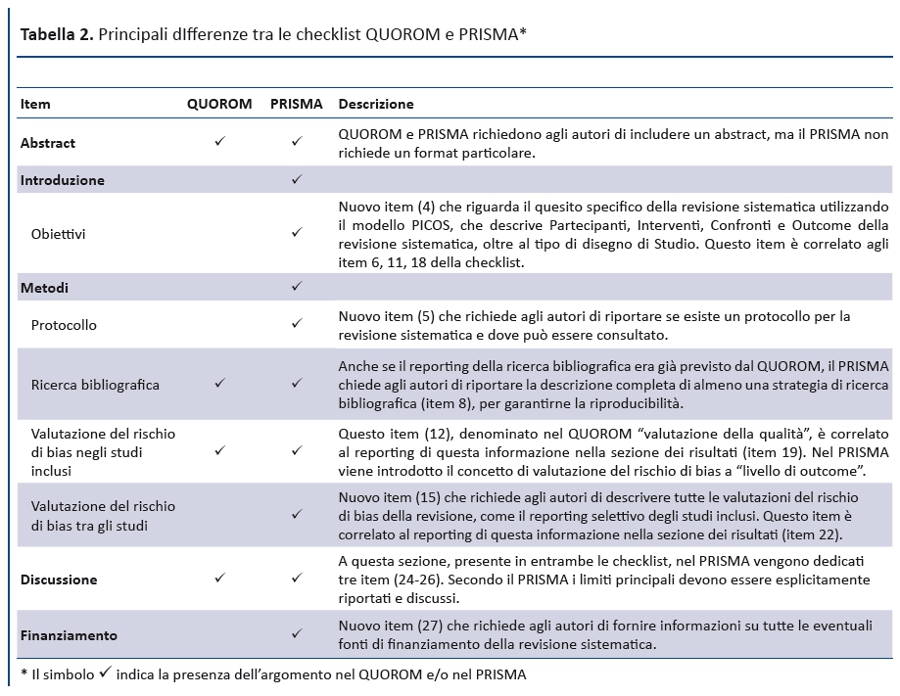
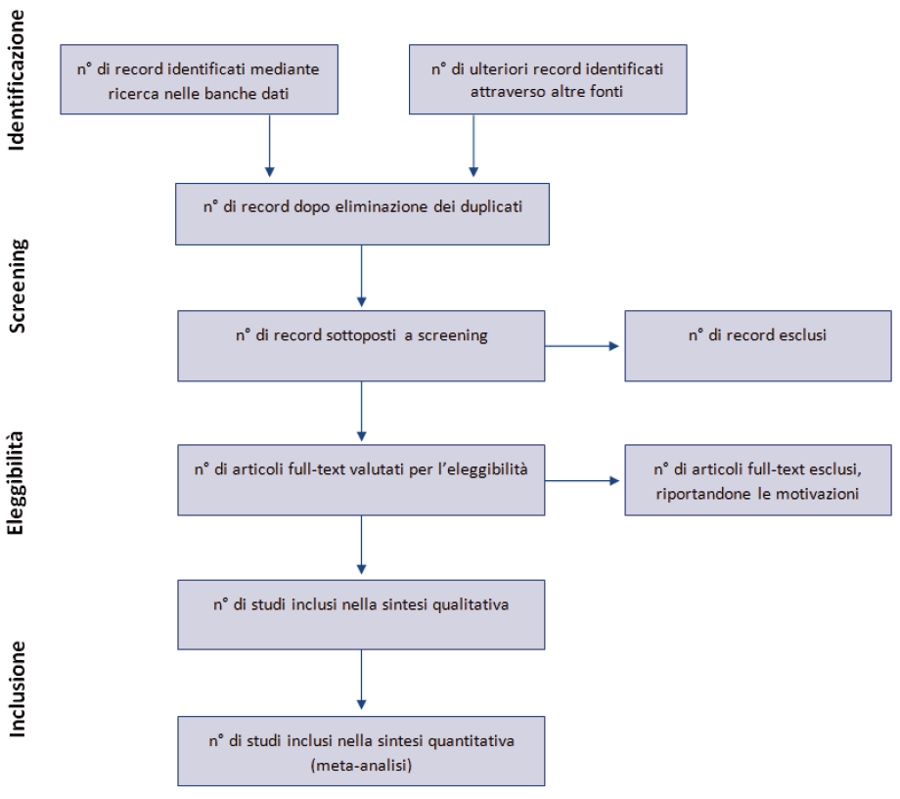
Linee guida per il reporting di revisioni sistematiche e meta-analisi: il PRISMA Statement
Guidelines & Standards
Linee guida per il reporting di revisioni sistematiche e meta-analisi: il PRISMA Statement
David Moher, Alessandro Liberati, Jennifer Tetzlaff, Douglas G. Altman, The PRISMA GroupEvidence 2015;7(6): e1000114 doi: 10.4470/E1000114
Pubblicato: 27 giugno 2015
Copyright: © 2015 Moher et al. Questo è un articolo open-access, distribuito con licenza Creative Commons Attribution, che ne consente l’utilizzo, la distribuzione e la riproduzione su qualsiasi supporto esclusivamente per fini non commerciali, a condizione di riportare sempre autore e citazione originale.
Vedi anche: PRISMA Statement per il reporting di revisioni sistematiche e meta-analisi degli studi che valutano gli interventi sanitari: spiegazione ed elaborazione
1. Oxman AD, Cook DJ, Guyatt GH. Users’ guides to the medical literature. VI. How to use an overview. Evidence-Based Medicine Working Group. JAMA 1994;272:1367-1371.
2. Swingler GH, Volmink J, Ioannidis JP. Number of published systematic reviews and global burden of disease: Database analysis. BMJ 2003;327:1083-1084.
3. Canadian Institutes of Health Research. Randomized controlled trials registration/application checklist (12/2006). Disponibile a: www.cihr-irsc.gc.ca/e/documents/rct_reg_e.pdf. Ultimo accesso: 27 giugno 2015.
4. Young C, Horton R. Putting clinical trials into context. Lancet 2005;366:107.
5. Mulrow CD. The medical review article: State of the science. Ann Intern Med 1985;106:485-488.
6. Sacks HS, Berrier J, Reitman D, Ancona-Berk VA, Chalmers TC. Metaanalysis of randomized controlled trials. New Engl J Med 1987;316:450-455.
7. Sacks HS, Reitman D, Pagano D, Kupelnick B. Meta-analysis: An update. Mt Sinai J Med 1996;63:216-224.
8. Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, et al. Improving the quality of reporting of meta-analysis of randomized controlled trials: The QUOROM statement. Lancet 1994;354:1896-1900.
9. Glossary of Cochrane terms. Disponibile a: http://community.cochrane.org/glossary. Ultimo accesso 27 giugno 2015.
10. Strech D, Tilburt J. Value judgments in the analysis and synthesis of evidence. J Clin Epidemiol 61: 521-524.
11. Moher D, Tsertsvadze A. Systematic reviews: When is an update an update? Lancet 2006;367:881-883.
12. University of York. Centre for Reviews and Dissemination. Disponibile a: www.york.ac.uk/inst/crd. Ultimo accesso 27 giugno 2015.
13. The Joanna Briggs Institute. Protocols & work in progress. Disponibile a: http://joannabriggs.org. Ultimo accesso 27 giugno 2015.
14. De Angelis C, Drazan JM, Frizelle FA, Haug C, Hoey J, et al. Clinical trial registration: A statement from the International Committee of Medical Journal Editors. CMAJ 2004;171:606-607.
15. Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, et al. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004;363:1341-1345.
16. Bagshaw SM, McAlister FA, Manns BJ, Ghali WA. Acetylcysteine in the prevention of contrast-induced nephropathy: A case study of the pitfalls in the evolution of evidence. Arch Intern Med 2006;166:161-166.
17. Biondi-Zoccai GG, Lotrionte M, Abbate A, Testa L, Remigi E, et al. Compliance with QUOROM and quality of reporting of overlapping metaanalyses on the role of acetylcysteine in the prevention of contrast associated nephropathy: Case study. BMJ 2006;332:202-209.
18. Altman DG, Schulz KR, Moher D, Egger M, Davidoff F, et al. The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann Intern Med 2001;134:663-694.
19. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD explanation and elaboration. Ann Intern Med 2003;138:W1-W12.
20. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med 2007;147:W163-W194.
21. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche P, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 2009;6:e1000100.
22. Moher D, Tetzlaff J, Tricco AC, Sampson M, Altman DG. Epidemiology and reporting characteristics of systematic reviews. PLoS Med 2007;4:e78.
23. Bhandari M, Morrow F, Kulkarni AV, Tornetta P. Meta-analyses in orthopaedic surgery: a systematic review of their methodologies. J Bone Joint Surg Am 2001;83-A:15-24.
24. Kelly KD, Travers A, Dorgan M, Slater L, Rowe BH. Evaluating the quality of systematic reviews in the emergency medicine literature. Ann Emerg Med 2001;38:518-526.
25. Richards D. The quality of systematic reviews in dentistry. Evid Based Dent 2004;5:17.
26. Choi PT, Halpern SH, Malik N, Jadad AR, Tramer MR, et al. Examining the evidence in anesthesia literature: a critical appraisal of systematic reviews. Anesth Analg 2001;92:700-709.
27. Delaney A, Bagshaw SM, Ferland A, Manns B, Laupland KB. A systematic evaluation of the quality of meta-analyses in the critical care literature. Crit Care 2005;9:R575–R582.
28. Dickersin K Publication bias: Recognizing the problem, understanding its origins and scope, and preventing harm. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication bias in meta-analysis-Prevention, assessment and adjustments. Chichester (UK): John Wiley & Sons, 2005:11–33.
29. Sutton AJ. Evidence concerning the consequences of publication and related biases. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication bias in meta-analysis-Prevention, assessment and adjustments. Chichester (UK): John Wiley & Sons, 2005:175-192.
30. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I- The case of the misleading funnel plot. BMJ 2006;333:597-600.
31. Ladabaum U, Chopra CL, Huang G, Scheiman JM, Chernew ME, et al. Aspirin as an adjunct to screening for prevention of sporadic colorectal cancer: a cost-effectiveness analysis. Ann Intern Med 2001;135:769-781.
32. Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ 2001;323:157-162.
33. Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224-228.
34. Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet 2001;29:306-309.
35. Lavis J, Davies H, Oxman A, Denis J, Golden-Biddle K, et al. Towards systematic reviews that inform health care management and policy-making. J Health Serv Res Policy 2005;10:35-48.
36. Stewart LA, Clarke MJ. Practical methodology of meta-analyses (overviews) using updated individual patient data. Cochrane Working Group. Stat Med 1995;14:2057-2079.
37. Moja LP, Telaro E, D’Amico R, Moschetti I, Coe L, et al. Assessment of methodological quality of primary studies by systematic reviews: Results of the metaquality cross sectional study. BMJ 2005;330: 1053-1055.
38. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-926.
39. Schunemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, et al. An official ATS statement: Grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med 2006;174:605-614.
40. Chan AW, Hrobjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291:2457-2465.
41. Chan AW, Krleza-Jeric K, Schmid I, Altman DG. Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research. CMAJ 2004;171:735-740.
42. Silagy CA, Middleton P, Hopewell S. Publishing protocols of systematic reviews: Comparing what was done to what was planned. JAMA 2002;287:2831-2834.
2. Swingler GH, Volmink J, Ioannidis JP. Number of published systematic reviews and global burden of disease: Database analysis. BMJ 2003;327:1083-1084.
3. Canadian Institutes of Health Research. Randomized controlled trials registration/application checklist (12/2006). Disponibile a: www.cihr-irsc.gc.ca/e/documents/rct_reg_e.pdf. Ultimo accesso: 27 giugno 2015.
4. Young C, Horton R. Putting clinical trials into context. Lancet 2005;366:107.
5. Mulrow CD. The medical review article: State of the science. Ann Intern Med 1985;106:485-488.
6. Sacks HS, Berrier J, Reitman D, Ancona-Berk VA, Chalmers TC. Metaanalysis of randomized controlled trials. New Engl J Med 1987;316:450-455.
7. Sacks HS, Reitman D, Pagano D, Kupelnick B. Meta-analysis: An update. Mt Sinai J Med 1996;63:216-224.
8. Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, et al. Improving the quality of reporting of meta-analysis of randomized controlled trials: The QUOROM statement. Lancet 1994;354:1896-1900.
9. Glossary of Cochrane terms. Disponibile a: http://community.cochrane.org/glossary. Ultimo accesso 27 giugno 2015.
10. Strech D, Tilburt J. Value judgments in the analysis and synthesis of evidence. J Clin Epidemiol 61: 521-524.
11. Moher D, Tsertsvadze A. Systematic reviews: When is an update an update? Lancet 2006;367:881-883.
12. University of York. Centre for Reviews and Dissemination. Disponibile a: www.york.ac.uk/inst/crd. Ultimo accesso 27 giugno 2015.
13. The Joanna Briggs Institute. Protocols & work in progress. Disponibile a: http://joannabriggs.org. Ultimo accesso 27 giugno 2015.
14. De Angelis C, Drazan JM, Frizelle FA, Haug C, Hoey J, et al. Clinical trial registration: A statement from the International Committee of Medical Journal Editors. CMAJ 2004;171:606-607.
15. Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, et al. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004;363:1341-1345.
16. Bagshaw SM, McAlister FA, Manns BJ, Ghali WA. Acetylcysteine in the prevention of contrast-induced nephropathy: A case study of the pitfalls in the evolution of evidence. Arch Intern Med 2006;166:161-166.
17. Biondi-Zoccai GG, Lotrionte M, Abbate A, Testa L, Remigi E, et al. Compliance with QUOROM and quality of reporting of overlapping metaanalyses on the role of acetylcysteine in the prevention of contrast associated nephropathy: Case study. BMJ 2006;332:202-209.
18. Altman DG, Schulz KR, Moher D, Egger M, Davidoff F, et al. The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann Intern Med 2001;134:663-694.
19. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD explanation and elaboration. Ann Intern Med 2003;138:W1-W12.
20. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med 2007;147:W163-W194.
21. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche P, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 2009;6:e1000100.
22. Moher D, Tetzlaff J, Tricco AC, Sampson M, Altman DG. Epidemiology and reporting characteristics of systematic reviews. PLoS Med 2007;4:e78.
23. Bhandari M, Morrow F, Kulkarni AV, Tornetta P. Meta-analyses in orthopaedic surgery: a systematic review of their methodologies. J Bone Joint Surg Am 2001;83-A:15-24.
24. Kelly KD, Travers A, Dorgan M, Slater L, Rowe BH. Evaluating the quality of systematic reviews in the emergency medicine literature. Ann Emerg Med 2001;38:518-526.
25. Richards D. The quality of systematic reviews in dentistry. Evid Based Dent 2004;5:17.
26. Choi PT, Halpern SH, Malik N, Jadad AR, Tramer MR, et al. Examining the evidence in anesthesia literature: a critical appraisal of systematic reviews. Anesth Analg 2001;92:700-709.
27. Delaney A, Bagshaw SM, Ferland A, Manns B, Laupland KB. A systematic evaluation of the quality of meta-analyses in the critical care literature. Crit Care 2005;9:R575–R582.
28. Dickersin K Publication bias: Recognizing the problem, understanding its origins and scope, and preventing harm. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication bias in meta-analysis-Prevention, assessment and adjustments. Chichester (UK): John Wiley & Sons, 2005:11–33.
29. Sutton AJ. Evidence concerning the consequences of publication and related biases. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication bias in meta-analysis-Prevention, assessment and adjustments. Chichester (UK): John Wiley & Sons, 2005:175-192.
30. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I- The case of the misleading funnel plot. BMJ 2006;333:597-600.
31. Ladabaum U, Chopra CL, Huang G, Scheiman JM, Chernew ME, et al. Aspirin as an adjunct to screening for prevention of sporadic colorectal cancer: a cost-effectiveness analysis. Ann Intern Med 2001;135:769-781.
32. Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ 2001;323:157-162.
33. Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224-228.
34. Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet 2001;29:306-309.
35. Lavis J, Davies H, Oxman A, Denis J, Golden-Biddle K, et al. Towards systematic reviews that inform health care management and policy-making. J Health Serv Res Policy 2005;10:35-48.
36. Stewart LA, Clarke MJ. Practical methodology of meta-analyses (overviews) using updated individual patient data. Cochrane Working Group. Stat Med 1995;14:2057-2079.
37. Moja LP, Telaro E, D’Amico R, Moschetti I, Coe L, et al. Assessment of methodological quality of primary studies by systematic reviews: Results of the metaquality cross sectional study. BMJ 2005;330: 1053-1055.
38. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-926.
39. Schunemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, et al. An official ATS statement: Grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med 2006;174:605-614.
40. Chan AW, Hrobjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291:2457-2465.
41. Chan AW, Krleza-Jeric K, Schmid I, Altman DG. Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research. CMAJ 2004;171:735-740.
42. Silagy CA, Middleton P, Hopewell S. Publishing protocols of systematic reviews: Comparing what was done to what was planned. JAMA 2002;287:2831-2834.