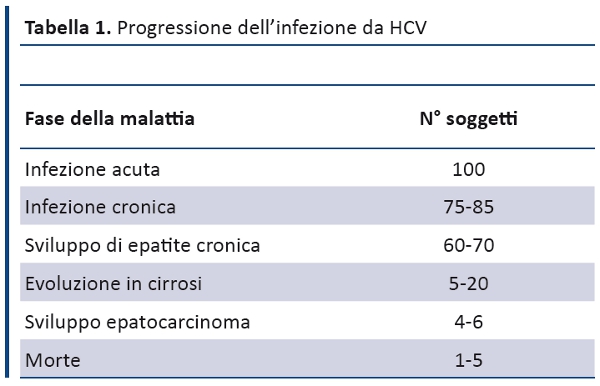
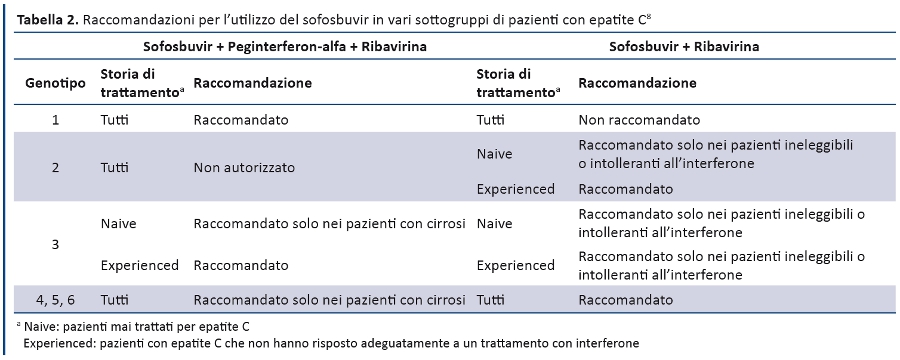
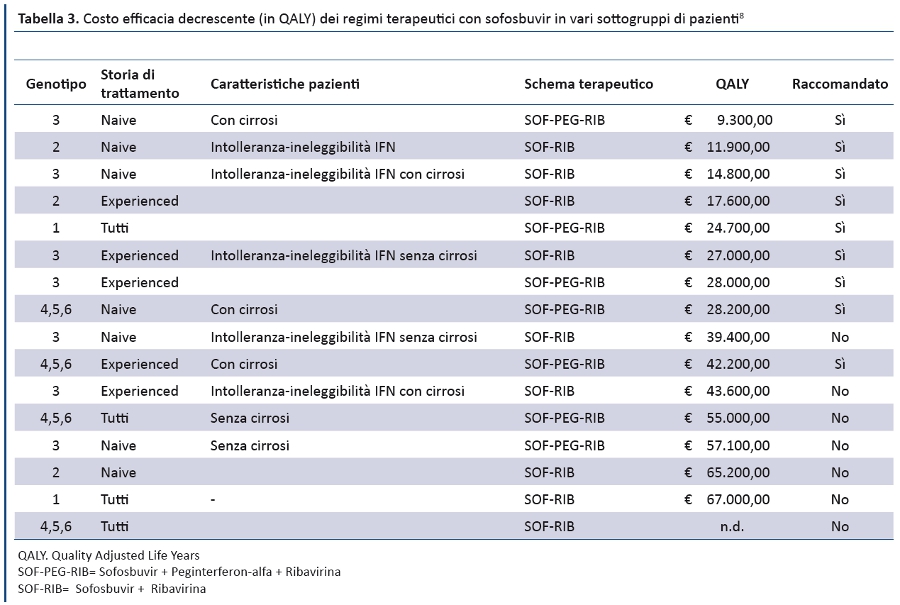
Position Statement GIMBE
Efficacia e costo-efficacia del sofosbuvir nel trattamento dell’epatite C
Antonino CartabellottaEvidence 2015;7(5): e1000111 doi: 10.4470/E1000111
Pubblicato: 26 maggio 2015
Copyright: © 2015 Cartabellotta. Questo è un articolo open-access, distribuito con licenza Creative Commons Attribution, che ne consente l’utilizzo, la distribuzione e la riproduzione su qualsiasi supporto esclusivamente per fini non commerciali, a condizione di riportare sempre autore e citazione originale.
1. Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet 2015;385:1124-35.
2. Centers for Disease Control and Prevention. Hepatitis C FAQs for Health Professionals. Disponibile a: www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Ultimo accesso 25 maggio 2015.
3. Institute for Quality and Efficiency in Health Care (IQWiG). Sofosbuvir – Benefit assessment according to §35a Social Code Book V. 29 April 2014.
4. Institute for Clinical and Economic Review (ICER). The Comparative Clinical Effectiveness and Value of Simeprevir and Sofosbuvir in the Treatment of Chronic Hepatitis C Infection. A Technology Assessment. Final Report: April 15, 2014
5. NIHR Horizon Scanning Centre. Sofosbuvir (Sovaldi) with GS-5816 for chronic hepatitis C. January, 2015
6. Canadian Agency for Drugs and Technologies in Health (CADTH). Common Drug Review. Sofosbuvir (Sovaldi — Gilead Sciences Canada, Inc.). Indication: Chronic Hepatitis C Infection. August 18, 2014.
7. Blue Cross Blue Shield Association. Cost-Effectiveness Studies of New Hepatitis C Treatments. January 2015.
8. National Institute for Health and Care Excellence (NICE). Sofosbuvir for treating chronic hepatitis. NICE technology appraisal guidance 330. February 2015
9. Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA 2014;312:631-40.
10. Canadian Agency for Drugs and Technologies in Health (CADTH). Sofosbuvir (Sovaldi): Sofosbuvir is indicated for the treatment of chronic hepatitis C virus (CHC) infection in adult patients with compensated liver disease, including cirrhosis Ottawa (ON, Canada: Canadian Agency for Drugs and Technologies in Health. Common Drug Review. 2014
11. Bansal S, Singal AK, McGuire BM, Anand BS. Impact of all oral anti-hepatitis C virus therapy: A meta-analysis. World J Hepatol 2015;7:806-13.
12. Interferon-free Regimens for Genotype 1 Chronic Hepatitis C: A Review of the Clinical Evidence and Cost-Effectiveness. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2014 Jun 25.
13. Druyts E, Lorenzi M, Toor K, Thorlund K, Mills EJ. Network meta-analysis of direct-acting antivirals in combination with peginterferon-ribavirin for previously untreated patients with hepatitis C genotype 1 infection. QJM 2015;108:299-306.
14. Liu X, Wang Y, Zhang G, Li N, Zhu Q, Chang H, Han Q, Lv Y, Liu Z. Efficacy and safety of sofosbuvir-based therapy for the treatment of chronic hepatitis C in treatment-naĂŻve and treatment-experienced patients. Int J Antimicrob Agents 2014;44:145-51.
15. Bucher HC, Guyatt GH, Cook DJ, Holbrook A, McAlister FA. Users’ guides to the medical literature: XIX. Applying clinical trial results. A. How to use an article measuring the effect of an intervention on surrogate end points. Evidence-Based Medicine Working Group. JAMA 1999;282:771-8.
16. Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med 1996;125:605-13.
17. Ciani O, Taylor RS. A more evidence based approach to the use of surrogate end points in policy making. BMJ 2011;343:d6498.
18. Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ 2011;343:d7995.
19. Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Stat Med 2012;31:2973-84.
20. Ciani O, Buyse M, Garside R, Pavey T, Stein K, Sterne JA, Taylor RS. Comparison of treatment effect sizes associated with surrogate and final patient relevant outcomes in randomised controlled trials: meta-epidemiological study. BMJ 2013;346:f457.
21. van der Meer AJ, Wedemeyer H, Feld JJ, Hansen BE, Manns MP, Zeuzem S, Janssen HL. Is there sufficient evidence to recommend antiviral therapy in hepatitis C? J Hepatol 2014;60:191-6.
22. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013;158(5 Pt 1):329-37.
23. Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, Lee WM, Lok AS, Bonkovsky HL, Morgan TR, Ghany MG, Morishima C, Snow KK, Dienstag JL; HALT-C Trial Investigators. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med 2008;359:2429-41.
24. Koretz RL, Pleguezuelo M, Arvaniti V, Barrera Baena P, Ciria R, Gurusamy KS, Davidson BR, Burroughs AK. Interferon for interferon nonresponding and relapsing patients with chronic hepatitis C. Cochrane Database Syst Rev 2013;1:CD003617.
25. Sofosbuvir (Sovaldi°). Active against hepatitis C virus, but evaluation is incomplete. Prescrire Int 2015;24:5-10.
26. No compelling reason to adopt new antivirals whenever it is reasonable to wait. Prescrire Int 2015;24:8.
2. Centers for Disease Control and Prevention. Hepatitis C FAQs for Health Professionals. Disponibile a: www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Ultimo accesso 25 maggio 2015.
3. Institute for Quality and Efficiency in Health Care (IQWiG). Sofosbuvir – Benefit assessment according to §35a Social Code Book V. 29 April 2014.
4. Institute for Clinical and Economic Review (ICER). The Comparative Clinical Effectiveness and Value of Simeprevir and Sofosbuvir in the Treatment of Chronic Hepatitis C Infection. A Technology Assessment. Final Report: April 15, 2014
5. NIHR Horizon Scanning Centre. Sofosbuvir (Sovaldi) with GS-5816 for chronic hepatitis C. January, 2015
6. Canadian Agency for Drugs and Technologies in Health (CADTH). Common Drug Review. Sofosbuvir (Sovaldi — Gilead Sciences Canada, Inc.). Indication: Chronic Hepatitis C Infection. August 18, 2014.
7. Blue Cross Blue Shield Association. Cost-Effectiveness Studies of New Hepatitis C Treatments. January 2015.
8. National Institute for Health and Care Excellence (NICE). Sofosbuvir for treating chronic hepatitis. NICE technology appraisal guidance 330. February 2015
9. Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA 2014;312:631-40.
10. Canadian Agency for Drugs and Technologies in Health (CADTH). Sofosbuvir (Sovaldi): Sofosbuvir is indicated for the treatment of chronic hepatitis C virus (CHC) infection in adult patients with compensated liver disease, including cirrhosis Ottawa (ON, Canada: Canadian Agency for Drugs and Technologies in Health. Common Drug Review. 2014
11. Bansal S, Singal AK, McGuire BM, Anand BS. Impact of all oral anti-hepatitis C virus therapy: A meta-analysis. World J Hepatol 2015;7:806-13.
12. Interferon-free Regimens for Genotype 1 Chronic Hepatitis C: A Review of the Clinical Evidence and Cost-Effectiveness. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2014 Jun 25.
13. Druyts E, Lorenzi M, Toor K, Thorlund K, Mills EJ. Network meta-analysis of direct-acting antivirals in combination with peginterferon-ribavirin for previously untreated patients with hepatitis C genotype 1 infection. QJM 2015;108:299-306.
14. Liu X, Wang Y, Zhang G, Li N, Zhu Q, Chang H, Han Q, Lv Y, Liu Z. Efficacy and safety of sofosbuvir-based therapy for the treatment of chronic hepatitis C in treatment-naĂŻve and treatment-experienced patients. Int J Antimicrob Agents 2014;44:145-51.
15. Bucher HC, Guyatt GH, Cook DJ, Holbrook A, McAlister FA. Users’ guides to the medical literature: XIX. Applying clinical trial results. A. How to use an article measuring the effect of an intervention on surrogate end points. Evidence-Based Medicine Working Group. JAMA 1999;282:771-8.
16. Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med 1996;125:605-13.
17. Ciani O, Taylor RS. A more evidence based approach to the use of surrogate end points in policy making. BMJ 2011;343:d6498.
18. Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ 2011;343:d7995.
19. Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Stat Med 2012;31:2973-84.
20. Ciani O, Buyse M, Garside R, Pavey T, Stein K, Sterne JA, Taylor RS. Comparison of treatment effect sizes associated with surrogate and final patient relevant outcomes in randomised controlled trials: meta-epidemiological study. BMJ 2013;346:f457.
21. van der Meer AJ, Wedemeyer H, Feld JJ, Hansen BE, Manns MP, Zeuzem S, Janssen HL. Is there sufficient evidence to recommend antiviral therapy in hepatitis C? J Hepatol 2014;60:191-6.
22. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013;158(5 Pt 1):329-37.
23. Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, Lee WM, Lok AS, Bonkovsky HL, Morgan TR, Ghany MG, Morishima C, Snow KK, Dienstag JL; HALT-C Trial Investigators. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med 2008;359:2429-41.
24. Koretz RL, Pleguezuelo M, Arvaniti V, Barrera Baena P, Ciria R, Gurusamy KS, Davidson BR, Burroughs AK. Interferon for interferon nonresponding and relapsing patients with chronic hepatitis C. Cochrane Database Syst Rev 2013;1:CD003617.
25. Sofosbuvir (Sovaldi°). Active against hepatitis C virus, but evaluation is incomplete. Prescrire Int 2015;24:5-10.
26. No compelling reason to adopt new antivirals whenever it is reasonable to wait. Prescrire Int 2015;24:8.